05680
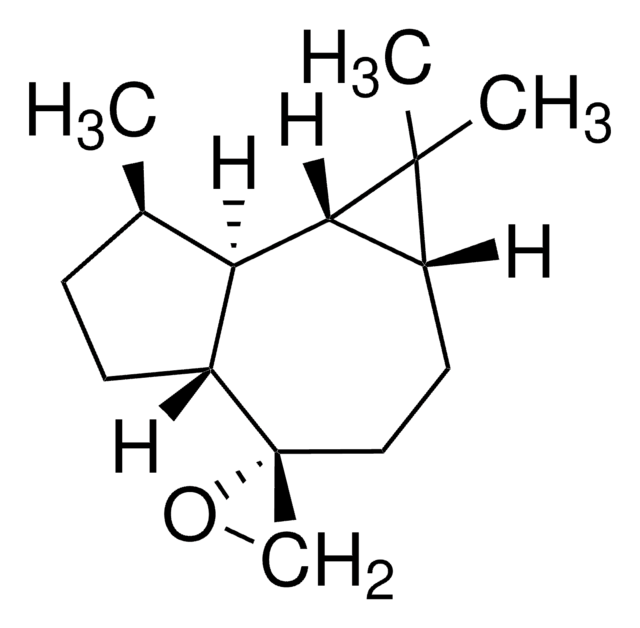
(−)-Alloaromadendrene
≥98.0% (sum of enantiomers, GC)
Synonym(s):
(−)-allo-Aromadendrene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H24
CAS Number:
Molecular Weight:
204.35
Beilstein/REAXYS Number:
2357213
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
assay
≥98.0% (sum of enantiomers, GC)
optical activity
[α]20/D −33±1°, neat
refractive index
n20/D 1.501
bp
265-267 °C (lit.)
density
0.923 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
C[C@@H]1CC[C@H]2[C@@H]1[C@H]3[C@@H](CCC2=C)C3(C)C
InChI
1S/C15H24/c1-9-6-8-12-14(15(12,3)4)13-10(2)5-7-11(9)13/h10-14H,1,5-8H2,2-4H3/t10-,11-,12-,13-,14-/m1/s1
InChI key
ITYNGVSTWVVPIC-DHGKCCLASA-N
Application
Alloaromadendrene can be used to study chiral building blocks, complex molecules, chemical syntheses and asymmetric syntheses. Alloaromadendrene has been used to apply headspace solid-phase microextraction (HS-SPME) coupled with GC/FID and GC/MS to the analysis of the volatile fraction of an Ephedra species. Alloaromadendrene has also been used to examine the temporal changes of methyl eugenol metabolites in Bactrocera correcta (guava fruit fly) male rectal glands.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
248.0 °F - closed cup
flash_point_c
120.00 °C - closed cup
ppe
Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jerome Niogret et al.
PloS one, 8(9), e73601-e73601 (2013-09-17)
Chemical analyses were conducted to determine the qualitative and quantitative differences in monoterpenes and sesquiterpenes in plant material from avocado trees, Persea americana Mill. (Lauraceae). The initial study analyzed plant material sampled from the trunk to the leaves through different
Anthony Giampetruzzi et al.
Molecular and cellular neurosciences, 56, 333-341 (2013-07-31)
Fragile X syndrome (FXS) is caused by lack of expression of fragile X mental retardation protein (FMRP), the product of the Fmr1 gene. In many cases FXS is associated with abnormalities in CNS myelination. Although FMRP is expressed in oligodendrocyte
Isao Tokushima et al.
Journal of chemical ecology, 36(12), 1327-1334 (2010-10-23)
The guava fruit fly, Bactrocera correcta, is widely distributed in Thailand and other surrounding Southeast Asian countries, and, like the closely related sympatric species, the oriental fruit fly, B. dorsalis, infests various fruits, including guava, peach, and mango. Males of
Filippo Maggi et al.
Chemistry & biodiversity, 8(1), 95-114 (2011-01-25)
Headspace solid-phase microextraction (HS-SPME) coupled with GC/FID and GC/MS was applied for the first time in the analysis of the volatile fraction of an Ephedra species. Notably, six Italian populations (Marche, Abruzzo, and Sardinia) of Ephedra nebrodensis subsp. nebrodensis, covering
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service