CRM05671
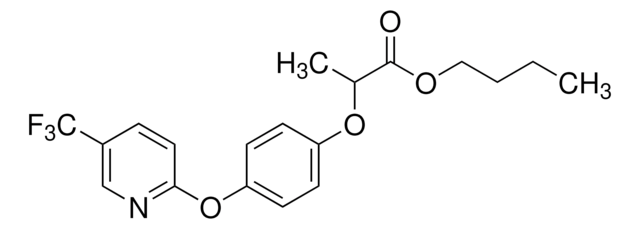
Fenoxaprop-P-ethyl
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
Synonym(s):
Ethyl (R)-2-{4-[(6-chlorobenzoxazol-2-yl)oxy]phenoxy}propionate
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
Quality Level
product line
TraceCERT®
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
storage temp.
2-8°C
SMILES string
CCOC(=O)[C@@H](C)Oc1ccc(Oc2nc3ccc(Cl)cc3o2)cc1
InChI
1S/C18H16ClNO5/c1-3-22-17(21)11(2)23-13-5-7-14(8-6-13)24-18-20-15-9-4-12(19)10-16(15)25-18/h4-11H,3H2,1-2H3/t11-/m1/s1
InChI key
PQKBPHSEKWERTG-LLVKDONJSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com
Fenoxaprop-P-ethyl (FPE) is a post-emergence herbicide that is used on many crops, such as rice, wheat, and barley, against annual and perennial grasses. It is an aryloxyphenoxypropionate that shows contact and systemic action. Fenoxaprop-P-ethyl works by inhibiting the activity of acetyl coenzyme A carboxylase and disrupting the synthesis of fatty acids. It is quickly converted to its metabolite fenoxaprop acid (FPA), another herbicidally active compound, in soil and plants. As per the commission regulation no 1107/2009, repealing the council directive 91/414/EEC unless otherwise stated the data available for fenoxaprop-P relates to its variant fenoxaprop-P-ethyl. It was approved for use in European Union on 1st January 2009 and the approval expires on 31st December 2022. According to Regulation (EC) no. 149/2008, a maximum residue limit (MRL) of 0.1 mg/kg applies for fenoxaprop-P in fresh/frozen fruits and tree nuts.
Application
The CRM may also find the following uses:
- Development and validation of gas chromatography (GC) and high-performance liquid chromatography (HPLC) method to detect FPE and its two primary metabolites in rice plants, rice straw, and rice grain
- Study of the degradation of FPE and its metabolite in soil and wheat crops by high-performance liquid chromatography (HPLC) coupled with UV detector
- Determination of FPE in barley and wheat grain samples using UV-Vis spectrophotometer
- Residue analysis of FPE and its metabolite fenoxaprop acid using HPLC in the study to evaluate the persistence of the herbicide and its metabolite in soil, wheat grain, and straw
- Development of a multi-residue enzyme-linked immunosorbent assay (ELISA) to detect two aryloxyphenoxypropionate herbicides in water, rice, soil, and soybean samples
- Direct determination of FPE from water and crop samples by voltametric method and also analysis of its reduction behavior on a hanging mercury drop electrode (HMDE)
Recommended products
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Skin Sens. 1 - STOT RE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service