464465
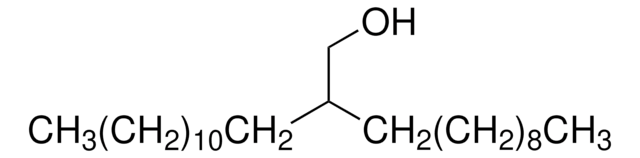
2-Butyl-1-octanol
95%
Synonym(s):
2-Butyloctanol, 2-Butyloctyl alcohol, 5-(Hydroxymethyl)undecane, Butyloctanol, Guerbet dodecanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)5CH[(CH2)3CH3]CH2OH
CAS Number:
Molecular Weight:
186.33
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
bp
145-149 °C (lit.)
density
0.833 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
CCCCCCC(CO)CCCC
InChI
1S/C12H26O/c1-3-5-7-8-10-12(11-13)9-6-4-2/h12-13H,3-11H2,1-2H3
InChI key
XMVBHZBLHNOQON-UHFFFAOYSA-N
General description
2-Butyl-1-octanol (BuOA) is a long-chain glass forming monohydroxy alcohol.
Application
2-Butyl-1-octanol (BuOA) has been used to synthesize:
It has also been used as an extraction solvent in extractive fed-batch experiments.
- 2-butyl-1-octyl-methacrylate (BOMA)
- 3,5,5-trimethyl-1-hexyl methacrylate (TMHMA)
- hydrophobic polyesters in miniemulsion in the presence of large amounts of water
It has also been used as an extraction solvent in extractive fed-batch experiments.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
251.6 °F - Non-equilibrium method
Flash Point(C)
122 °C - Non-equilibrium method
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gergely Kali et al.
Langmuir : the ACS journal of surfaces and colloids, 23(21), 10746-10755 (2007-09-11)
Seven amphiphilic conetworks of methacrylic acid (MAA) and a new hydrophobic monomer, 2-butyl-1-octyl-methacrylate (BOMA), were synthesized using group transfer polymerization. The MAA units were introduced via the polymerization of tetrahydropyranyl methacrylate (THPMA) followed by the removal of the protecting tetrahydropyranyl
Helena González-Peñas et al.
Biotechnology letters, 37(3), 577-584 (2014-10-30)
Acetone/butanol/ethanol (ABE) fermentation by Clostridium acetobutylicum was investigated in extractive fed-batch experiments. In conventional fermentations, metabolic activity ceases when a critical threshold products concentration is reached (~21.6 g solvents l(-1)). Solvents production was increased up to 36.6 and 37.2 g
Polyester synthesis in aqueous miniemulsion.
Barrere M and Landfester K.
Polymer, 44(10), 2833-2841 (2003)
Yanqin Gao et al.
The Journal of chemical physics, 139(16), 164504-164504 (2013-11-05)
The dielectric relaxation of two long-chain glass forming monohydroxy alcohols, 2-butyl-1-octanol and 2-hexyl-1-decanol, is studied at low temperature. Remarkable broadening from the pure Debye relaxation is identified for the slowest dynamics, differing from the dielectric spectra of short-chain alcohols. The
Liping Kong et al.
Bioresource technology, 299, 122582-122582 (2019-12-27)
Direct hydrogenolysis of Kraft lignin was catalyzed over a series of supported Ni or Re catalysts in ethanol solvent. The best results showed that the oil yield of 96.70 wt% was obtained with less char formation at 330 °C for 3 h over
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service