Recommended Products
Quality Level
product line
Novabiochem®
form
beads
reaction suitability
reaction type: solution phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
storage temp.
2-8°C
Related Categories
General description
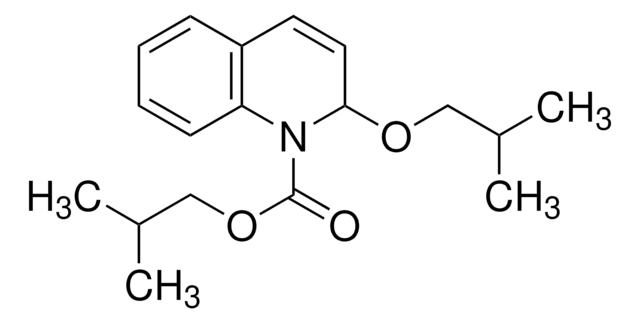
IIDQ-polystyrene (IIDQ-PS) [1] is a polymer-supported version of the IIDQ coupling reagent [2]. IIDQ has many advantages over conventional carbodiimide- or uronium-based reagents: no preactivation step is required, and acid, amine and coupling reagent can be added in any order; in contrast to uronium-based reagents like HBTU, it cannot form guanidinium by-products; and it is totally stable to base. The treatment of a carboxylic acid with IIDQ-PS in DCM or MeCN rapidly generates in situ the corresponding isobutoxycarbonyl mixed anhydride [3]. Attack by nucleophiles preferentially takes place at the less hindered and more electrophilic carbonyl of the carboxylic acid moiety, releasing only volatile carbon dioxide and isobutanol as by-products. If reaction is carried out in the presence of an amine, amide bond formation occurs concurrently with generation of the anhydride. Alternatively, addition of NaBH4 or polymer-supported borohydride to the anhydride will lead directly to the corresponding alcohol. IIDQ-PS appears to be particularly effective for mediating the acylation for anilines, and has also been found to couple peptide fragments without epimerization. In a comparative study, IIDQ-PS was found to give higher yields and greater purities than HATU, EDC-PS or DCC-PS [4]. Occasionally, with some secondary amines the formation of isobutyl carbamate by-products has been observed, resulting from attack by the amine at the carbonyl group.
Literature references
[1] E. Valeur, et al. (2005) Chem. Commun., 1164.
[2] Y. Kiso, et al. (1973) Chem. Pharm. Bull., 21, 2507.
[3] J. R. Vaughan (1951) J. Am. Chem. Soc., 73, 3547.
[4] E. Valeur & M. Bradley, unpublished results.
Literature references
[1] E. Valeur, et al. (2005) Chem. Commun., 1164.
[2] Y. Kiso, et al. (1973) Chem. Pharm. Bull., 21, 2507.
[3] J. R. Vaughan (1951) J. Am. Chem. Soc., 73, 3547.
[4] E. Valeur & M. Bradley, unpublished results.
Linkage
Replaces: 01-64-0469
Analysis Note
Color (visual): yellow to beige to amber
Appearance of substance (visual): beads
Loading (determined by HPLC after reaction of 3-phenylpropionic acid with cyclohexlamine): 1.3 - 1.9 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene-1 % DVB) 200 - 400 mesh.
Appearance of substance (visual): beads
Loading (determined by HPLC after reaction of 3-phenylpropionic acid with cyclohexlamine): 1.3 - 1.9 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene-1 % DVB) 200 - 400 mesh.
Legal Information
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service