Density Gradient
To meet highest demands for quality, we offer a wide range of BioUltra Reagents for density gradient centrifugation.
Introduction
Criteria for an ideal density gradient centrifugation medium are:
- the additive must form a solution within the required density range
- the additive must not interfere with, or damage, the sample
- the solvent must be compatible with the sample
- the solution must have a refractive index within the practical range, as well as a low viscosity
- the additive must be easily removable from the sample.
The additives for density gradient centrifugation can be divided into four main categories:
1. Salts of Alkali Metals
These solutions fulfill most of the above requirements. However, due to the high ionic strength, hydrogen bonding within biological macromolecules (protein, nucleic acid - protein complexes) is impaired by a chaotropic effect. Therefore these salts are mainly used for DNA and RNA separations. Cesium chloride is used most frequently. Other useful salts include sodium iodide, sodium bromide, cesium sulfate and cesium acetate. Potassium tartrate has been used to separate viruses from host cells.
It should be kept in mind that the density of the sample is highly dependent on the hydration of the macromolecule, which in turn depends to a large extent on the dehydration power of the salt solution.
2. Neutral, Water-Soluble Molecules
In this class of compounds sucrose is most widely used. It has a useful density range of up to 1.29. This range can be increased to 1.37 by addition of glucose or by dissolving sucrose in D2O. Sucrose has very little effect on macromolecules, but affects enzyme activity. Due to its high osmotic pressure, sucrose solution dehydrates cells and their organellae very efficiently. Glycerol solutions are the preferred media for the separation of enzymes because they do not affect enzyme activity. They exhibit a high viscosity, requiring prolonged centrifugation times. More importantly however, glycerol penetrates biological membranes.
3. Hydrophilic Macromolecules
Dextran gradients have been used for the separation of microsomes. Separations achieved with dextrans show similar results to those obtained by using synthetic sucrose/ epichlorohydrin co-polymers. In some cases bovine serum albumin has been applied, but the preparation of an appropriate solution is very difficult.
4. Synthetic Molecules
These additives are the sodium or methyl glucamine salt of triiodobenzoic acid and of metrizoic acid. It should be kept in mind that the parent acid of these salts may precipitate on adjusting the pH to acidic values. Metrizamide, a covalently bonded compound of glycosamine and metrizoic acid is most widely used. This additive forms solutions of relatively low viscosity. These solutions are stable over a wide range of pH and ionic strength, and show practically no interference with the analytes.
References:
R. Hinton M. Dobrota, in: Laboratory Techniques in Biochemistry and Molecular Biology (TW.Work, E.Work, ebs.), North Holland Publ.Comp., Amsterdam (1976).
Nomogram for Determination of g-number x
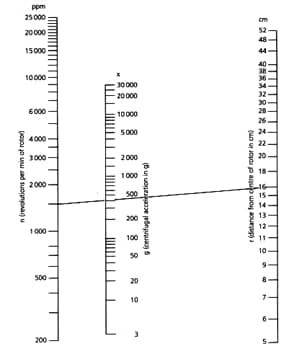
Formula(1) describes the dependence of the centrifugal field g (in g-numbers x) with revolutions per min, n, and with distance, r (in centimeters), from the midpoint of the rotor to the point at which the field is determined.
xg= 1118 x 10-8 x r x n2 (1)
(g= gravity accelaration of earth)
Properties of Aqueous Solutions of 84097 and 84099 Sucrose BioUltra
(Density, Refractive Index and Viscosity vs w/w-% sucrose)
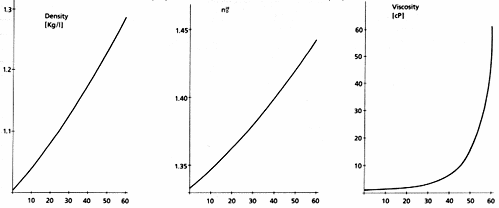
For explicit data on the viscosity and refractive index of aqueous solutions at various temperatures: D. Ridge, in: Centrifugal Separations in Molecular and Cell Biology (D. D. Birnie, D.Rickwood, eds.), p.33, Butterworth, London and Boston (1978) Barber, Natl. Cancer Inst. Monographs 2, 219 (1966)
Density of Gradient Centrifugation Media and their Useful Range calculated from n25 D |
|---|
Calculation of Density of Sucrose between 0° and 30 °C1
p (T) = (B1+ B2T+ B3T2) + (B4 + B5T+ B6T2)Y + (B7 + B8T + B9T2)Y2 (2)
T = Temperature °C
Y = weight fraction of sucrose in solution
B1-9 = constants
B1: 1.00037
B2: 3.96805x10-5
B3: -5.85133 x10-6
B4: 0.389824
B5 -1.05789x10-3
B6: 1.23928x10-5
B7: 0.170976
B8: 4.75301 x10-4
B9: -8.92397x10-6
Tables of density and refractive index vs w/w-% solutions of sucrose and CsCI can be found in.2
References
To continue reading please sign in or create an account.
Don't Have An Account?