670774
(R)-(−)-Pyrrolidine-3-carboxylic acid
≥99.0% (NT)
Synonym(s):
(R)-β-Proline
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H9NO2
CAS Number:
Molecular Weight:
115.13
Beilstein:
5496144
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
Assay
≥99.0% (NT)
form
solid
optical activity
[α]/D -20.5±1.5°, c = 2 in H2O
reaction suitability
reaction type: solution phase peptide synthesis
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
OC(=O)[C@@H]1CCNC1
InChI
1S/C5H9NO2/c7-5(8)4-1-2-6-3-4/h4,6H,1-3H2,(H,7,8)/t4-/m1/s1
InChI key
JAEIBKXSIXOLOL-SCSAIBSYSA-N
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cody Timmons et al.
The Journal of organic chemistry, 70(19), 7634-7639 (2005-09-10)
[reaction: see text] A new halo-Mannich-type reaction is reported using cyclopropyl carbonyl-derived enolates and sulfonyl-protected imines. Chiral oxazolidinones auxiliaries were found to be effective for completely controlling the stereochemistry of the products. Variations in the oxazolidinone, protecting group, and imine
Alan Armstrong et al.
The Journal of organic chemistry, 74(14), 5041-5048 (2009-06-03)
7-Azabicyclo[2.2.1]heptane-2-carboxylic acid 11 was prepared in enantiopure form, and its catalytic potential in the direct aldol reaction between acetone and 4-nitrobenzaldehyde was assessed. The bicyclic system was found to be more selective than its monocyclic analogue beta-proline 5b. A comparative
Haile Zhang et al.
Journal of the American Chemical Society, 130(3), 875-886 (2008-01-01)
The development of enantioselective anti-selective Mannich-type reactions of aldehydes and ketones with imines catalyzed by 3-pyrrolidinecarboxylic acid and related pyrrolidine derivatives is reported in detail. Both (3R,5R)-5-methyl-3-pyrrolidinecarboxylic acid and (R)-3-pyrrolidinecarboxylic acid efficiently catalyzed the reactions of aldehydes with alpha-imino esters
Giuliana Cardillo et al.
Journal of medicinal chemistry, 45(12), 2571-2578 (2002-05-31)
In this paper we describe the synthesis and affinity toward the mu-opioid receptor of some tetrapeptides obtained from endomorphin-1, H-Tyr-Pro-Trp-Phe-NH(2) (1), by substituting each amino acid in turn with its homologue. The ability to bind mu-opioid receptors depends on the
Souvik Banerjee et al.
The Journal of organic chemistry, 77(23), 10925-10930 (2012-11-07)
A straightforward stereoselective and enantiodivergent cyclization strategy for the construction of γ-lactams is described. The cyclization strategy makes use of chiral malonic esters prepared from enantiomerically enriched monoesters of disubstituted malonic acid. The cyclization occurs with the selective displacement of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service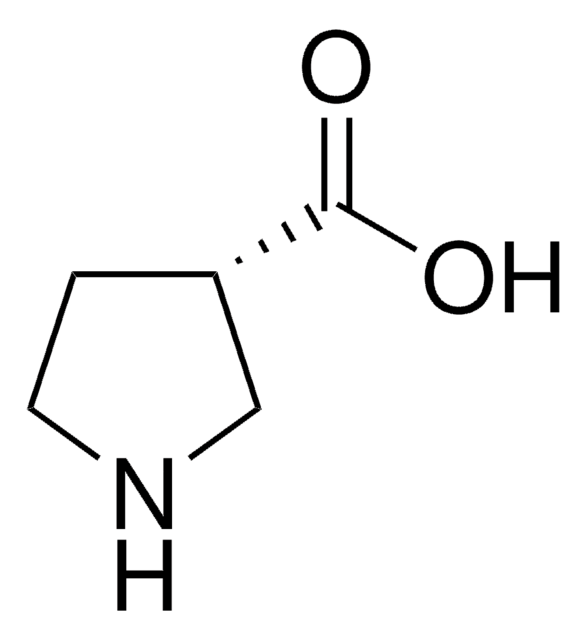




![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol ≥99.0%](/deepweb/assets/sigmaaldrich/product/structures/201/440/11d18670-8609-4657-bb4b-af6c424f8791/640/11d18670-8609-4657-bb4b-af6c424f8791.png)