D120006
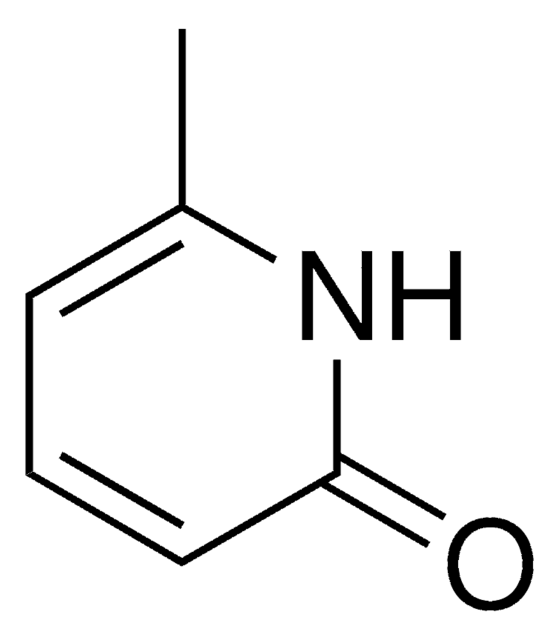
2,6-Dihydroxypyridine hydrochloride
97%
Synonym(s):
2,6-Pyridinediol hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5NO2 · HCl
CAS Number:
Molecular Weight:
147.56
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
206-208 °C (dec.) (lit.)
SMILES string
Cl[H].Oc1cccc(O)n1
InChI
1S/C5H5NO2.ClH/c7-4-2-1-3-5(8)6-4;/h1-3H,(H2,6,7,8);1H
InChI key
HNWWAWKDVFVJRG-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Paula Sachelaru et al.
Journal of bacteriology, 187(24), 8516-8519 (2005-12-03)
The enzyme catalyzing the hydrolytic cleavage of 2,6-dihydroxypseudooxynicotine to 2,6-dihydroxypyridine and gamma-N-methylaminobutyrate was found to be encoded on pAO1 of Arthrobacter nicotinovorans. The new enzyme answers an old question about nicotine catabolism and may be the first C--C bond hydrolase
H Stopper et al.
Biochemical and biophysical research communications, 203(2), 1124-1130 (1994-09-15)
The rate limiting step in 5-fluorouracil catabolism is catalyzed by the enzyme dihydropyrimidine dehydrogenase. Since degradation of 5-fluorouracil decreases its efficacy in chemotherapy, the inhibition of its catabolism is a promising tool. We investigated the formation of micronuclei in vitro
M Fukushima et al.
Gan to kagaku ryoho. Cancer & chemotherapy, 23(6), 721-731 (1996-05-01)
Possible pathways of intracellular phosphorylation of 5-fluorouracil (5-FU) in human cancer cells were investigated in vitro and in vivo. We used two inhibitors which regulate the anabolism of 5-FU for the purpose of elucidation of its pathways; one is oxonic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service