8.56000
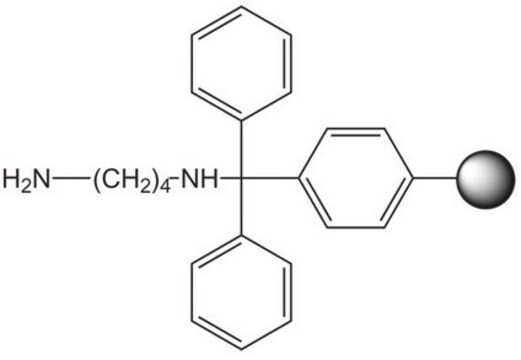
Cysteamine 2-chlorotrityl resin
Novabiochem®
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Code UNSPSC :
12352101
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Gamme de produits
Novabiochem®
Forme
beads
Capacité de réaction
reaction type: Fmoc solid-phase peptide synthesis
Fabricant/nom de marque
Novabiochem®
Application(s)
peptide synthesis
Groupe fonctionnel
amine
Température de stockage
2-8°C
Description générale
An acid-labile resin for the preparation of N-acyl or N-alkyl cysteamines. The free amino functionality of the resin-bound cysteamine can be readily acylated or reductively alkylated using standard procedures. Cleavage can be effected with electrophilic oxidants such as I2 or Tl(3) to produce a dimeric disulfide bridged product, or with 50-100% TFA to give the monomeric sulfhydryl product. Peptidylaminoethylthiols produced in this manner have been used to prepare PEG-conjugates by chemoselective ligation to pegylated maleimide [1]. This method is particularly useful for forming intramolecular disulfide bridges in molecules containing two thiol groups where one is protected with Acm. For a similar application, see [2,3,4].
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] J. Zhang, et al. Poster 162 presented at the 16 American Peptide Symposium, Minneapolis, 1999.
[2] A. v. Vliet, et al. in ′Innovation & Perspectives in Solid Phase Synthesis, 2nd International Symposium′, R. Epton (Eds), Intercept UK Ltd., Andover, 1992, pp. 475.
[3] A. v. Vliet, et al. in ′Peptides 1992, Proc.22nd European Peptide Symposium′, C. H. Schneider & A. N. Eberle (Eds), ESCOM, Leiden, 1993, pp. 279.
[4] A. v. Vliet, et al. in ′Peptides, Chemistry, Structure, &Biology, Proc. 13th American Peptide Symposium′, R. S. Hodges & J. A. Smith (Eds), ESCOM, Leiden, 1994, pp. 151.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] J. Zhang, et al. Poster 162 presented at the 16 American Peptide Symposium, Minneapolis, 1999.
[2] A. v. Vliet, et al. in ′Innovation & Perspectives in Solid Phase Synthesis, 2nd International Symposium′, R. Epton (Eds), Intercept UK Ltd., Andover, 1992, pp. 475.
[3] A. v. Vliet, et al. in ′Peptides 1992, Proc.22nd European Peptide Symposium′, C. H. Schneider & A. N. Eberle (Eds), ESCOM, Leiden, 1993, pp. 279.
[4] A. v. Vliet, et al. in ′Peptides, Chemistry, Structure, &Biology, Proc. 13th American Peptide Symposium′, R. S. Hodges & J. A. Smith (Eds), ESCOM, Leiden, 1994, pp. 151.
Liaison
Replaces: 01-64-0107
Remarque sur l'analyse
Color (visual): white to yellow to beige
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Leu loaded resin): 1.00 - 2.00 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene-1% DVB), 200 - 400 mesh.
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Leu loaded resin): 1.00 - 2.00 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene-1% DVB), 200 - 400 mesh.
Informations légales
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique