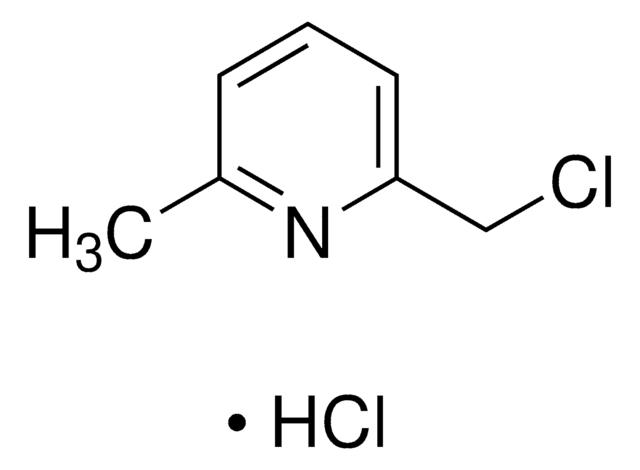
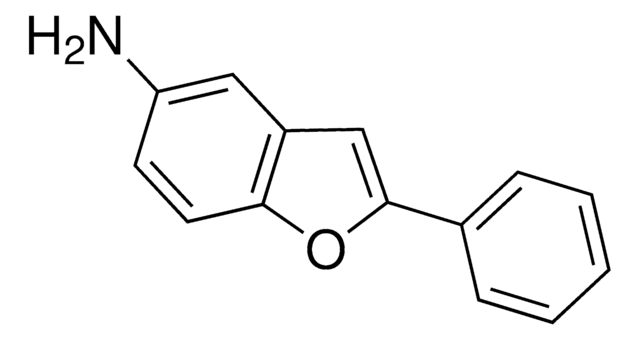
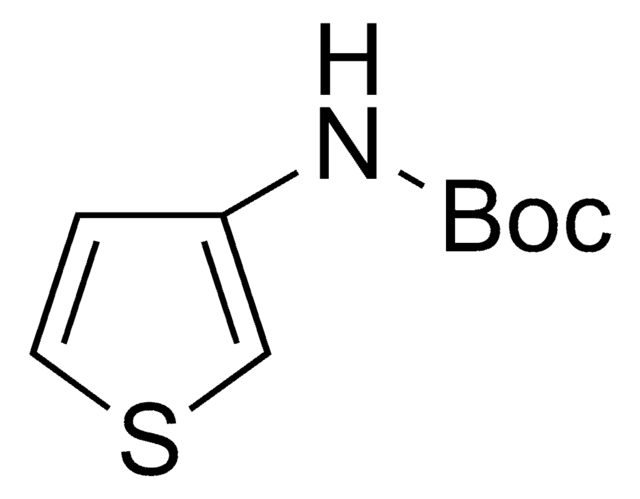
220884
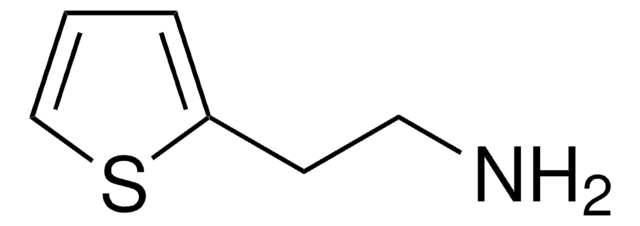
2-Thiophenemethylamine
96%
Synonyme(s) :
2-(Aminomethyl)thiophene
About This Item
Produits recommandés
Pureté
96%
Forme
liquid
liquid
Indice de réfraction
n20/D 1.5670 (lit.)
Point d'ébullition
95-99 °C/28 mmHg (lit.)
Densité
1.103 g/mL at 25 °C (lit.)
Chaîne SMILES
NCc1cccs1
InChI
1S/C5H7NS/c6-4-5-2-1-3-7-5/h1-3H,4,6H2
Clé InChI
FKKJJPMGAWGYPN-UHFFFAOYSA-N
Description générale
Application
- naphthalene-thiophene hybrid molecule (Z)-1-((thiophen-2-ylmethylamino)methylene)naphthalen-2(1H)-one
- fluorescent Pd2+ sensor, N-butyl-4-(p-methyloxy)-phenylethynyl-5-thiophenemethylamino-1,8-naphthalimide
- Triazole-linked-thiopene conjugates for use as a biomimetic model for studies of metal detoxification and oxidative stress involving metallothionein
- Serotonin 5-HT1A receptor antagonists which have neuroprotective affects against ischemic cell damage
- Imidazole- and piperonyl-containing thiadiazoles and pyrimidines for use as inducible oxide synthase dimerization inhibitors
- Optoelectronic segmented polyurethanes
Reactant involved in:
- Studies of organocatalyzed asymmetric reductive amination of ketones
- Metal-free aerobic oxidative coupling of amines to imines
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Skin Corr. 1B
Code de la classe de stockage
8A - Combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
165.2 °F - closed cup
Point d'éclair (°C)
74 °C - closed cup
Équipement de protection individuelle
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique