Protein Conformation Array Multiplexing
Understanding HOS for successful biosimilar mAb development
Monoclonal antibodies (mAbs) are the fastest growing class of human therapeutics within the field of biologics, having predicted worldwide sales of $125 billion by 2020 based on current approval rates. As the number of therapeutic mAbs reaching the market continues to rise, a corresponding increase in the number of biosimilars is expected.1
The US Food and Drug Administration (FDA) definition of a biosimilar describes a biological product that is highly similar to, and has no clinically meaningful differences from, an existing FDA-approved reference product.
The production of biosimilar mAbs is challenging since the three-dimensional (3D) structure, a key factor in the biological function of these molecules, is highly complex. Even small structural differences between a biosimilar mAb and the reference therapeutic mAb it is based upon can lead to significant impacts on the safety, efficacy and function of the molecule.
The 3D structure of a mAb arises from the combined effects of primary, secondary, tertiary and quaternary structure, in addition to the influence of posttranslational modifications. Referred to as the Higher Order Structure (HOS), this conformation is influenced not only by the underlying gene but also by factors including the choice of cell line for producing the biosimilar, bioprocessing conditions such as temperature, pH and light exposure, and the method of formulation.
With so many variables to consider, it is often the case that a biosimilar mAb is not identical to the product it is based upon. To avoid the multitude of issues that can occur as a result of these differences, FDA guidelines published in 2015 recommend that extensive, robust comparative physicochemical and functional studies should be performed to evaluate the biosimilarity of a proposed product to a reference product.2
Current methods for analyzing HOS are detailed below, yet while all of these provide useful information about the 3D conformation of a mAb, none of them offer both sensitivity and affordability:
- Circular Dichroism Spectroscopy
- Size Exclusion Chromatography (SEC)
- Analytical Ultracentrifugation (AUC)
- Non-denaturing Electrophoresis
- Hydrogen/deuterium exchange Mass Spectroscopy (HDX/MS)
- NMR Spectroscopy
Due to the limitations of these methods, and a drive to meet the requirements of the FDA, there has been an increasing need for an alternative and higher throughput approach to analyzing the HOS of biosimilar mAbs relative to a reference product. Through our acquisition of the commercial rights to exclusively distribute and research Protein Conformational Array (PCA) technology from Array Bridge Inc., MilliporeSigma provides researchers with a convenient solution to this problem. Located in close proximity to our St. Louis campus, Array Bridge Inc. are experienced in the measurement of biosimilar comparability at the molecular level.
Protein Conformational Array technology
Within their 2013 publication in Frontiers in Pharmacology, Wang et al of Array Bridge Inc. described the development of sophisticated antibody arrays for probing mAb HOS.3 By generating polyclonal antibodies (pAbs) against peptide immunogens with overlapping regions, covering the entire mAb structure, the authors demonstrated that different marketed mAbs possessed distinct HOS signatures despite sharing almost identical amino sequences.
The HOS signature represents observable changes in the different mAb species that are detected by the panel of pAbs due to chemical modifications and or physical changes to the antibody structure. To better understand this, it is important to recognize that each pAb within the panel binds the linear or secondary structure epitope, which may be inaccessible, partially or fully exposed when the mAb is in its correctly folded native form. Presentation of these epitopes on the surface of the mAb due to unfolding or misfolding results in a change of signal.
The information generated from these antibody arrays can be used to benchmark the original mAb, to analyze the biosimilar in comparison to this product, and to check for any conformational changes which may lead to clinically relevant effects of the new mAb.
PCA technology is extremely user-friendly. Run as an easy-to-operate ELISA format, the assays provide rapid, accurate and sensitive measurement of mAb HOS with utility across all stages of mAb development.

Distribution of the 34 antibodies in the Antibody Array. The pAb array employed by PCA technology covers the amino acid sequence of the entire mAb.
Utilizing PCA ELISAs to analyze HOS for process development
When establishing a mAb manufacturing process, various conditions require careful optimization. Changes originating from cell line engineering, media and additive selections, and process development may result in changes to the HOS of a therapeutic mab. The following data, generated using our RituBridge PCA ELISA, show the effects of a variety of harsh conditions – oxidation, heat and high pH - on the HOS of the mAb Rituxan, which is used to treat various forms of CD20-positive non-Hodgkin’s lymphoma. While all three conditions caused the mAb to unfold, the pattern of unfolding was markedly different in each case, giving rise to a different HOS fingerprint under each condition tested.

The effect of oxidation, heat and pH change on Rituxan, measured using the RituBridge PCA ELISA 1 (of 3). Spikes in optical density occur when normally inaccessible epitopes become exposed, and are indicative of changes in HOS.
Utilizing PCA ELISAs to analyze HOS for FDA submission
In addition to evaluating the effect of physicochemical conditions on a mAb, PCA ELISAs can also be used to compare a biosimilar to multiple lots of an originator molecule. The data below clearly illustrates excellent similarity of a candidate biosimilar to its innovator with only potential deviation at Ab region 9.
It is important to note that although a biosimilar may demonstrate a different HOS signature to the originator molecule, these changes may not always be clinically relevant. PCA ELISA data adds significant value to the evidence which is required to support an FDA submission.

Comparison of a biosimilar to five different lots of an originator molecule illustrates four regions (5, 9, 17 and 30) where performance deviances may be present.
InnoPlex for high-throughput PCA in novel mAb development
InnoPlex is a PCA that specifically targets novel therapeutic mAbs based on the conserved structure of IgG1 from a CHO cell line. While each of the PCA ELISA formats allows three compounds to be measured in parallel, we have increased this assay’s throughput to approximately 45 compounds per plate in duplicate by developing a multiplexed methodology known as InnoPlex.
InnoPlex incorporates Luminex® Corporation’s xMAP® technology with the InnoBridge ELISA to allow simultaneous detection of multiple analytes using specialized microspheres color-coded with varying combinations of dyes. By immobilizing the pAbs on the surface of these beads it is now possible to screen each of the 34 target epitopes concurrently within a single well of a 96-well plate.

Example data from the multiplexing of Ritubridge & InnoBridge to become RituPlex and InnoPlex, high-throughput PCAs.
Biosimilar Candidate Stressed Under Acidic and Basic Conditions
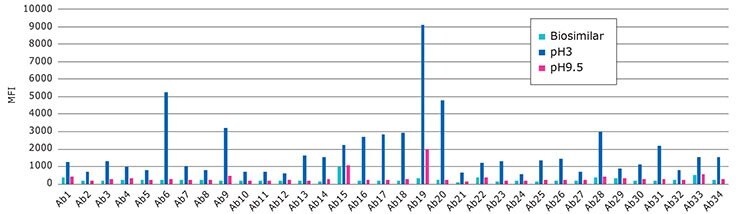
Performance of the RituBridge ELISA antibodies 1-34, multiplexed with xMAP technology (RituPlex), control known Biosimilar Mab treated at low pH.
Control vs. pH3

Change detected in a new therapeutic Mab (unknown biosimilar) under pH induced stress utilizing the INNOPLEX multiplex PCA assay on Luminex xMAP™ Technology.
The likelihood of therapeutic mAbs being immunogenic is an issue that has previously been well-documented, and the significance of this issue is exemplified in a 2018 publication by Song et al. Within their study the authors demonstrated the InnoPlex method to be suitable for HOS analysis in the development of therapeutic antibodies, generating PCA data which correlated well with protein stability results from traditional methods such as size-exclusion chromatography.5
The study authors also detected various structural differences between mAbs, with one important observation being that processing conditions resulted in antibody aggregation which caused significantly higher exposure to the antibody epitopes. They noted that this had the potential to lead to a “foreign” molecule which could cause immunogenicity.
References
To continue reading please sign in or create an account.
Don't Have An Account?