A New Thermal Desorption Tube for Air Sampling of Cannabis Terpenes

Introduction
There is a growing interest in sampling terpenes from the air.1,2,3 Since gas chromatography is the most common technique used for terpene analysis, a new thermal desorption (TD) tube specific for sampling terpenes relevant to cannabis and hemp was developed. This provides a means to monitor/determine terpene concentrations in the cultivation environment.
Thermal desorption is less wasteful and more environmentally friendly than single-use devices since the TD tubes can be reused and no (toxic) solvents are required for the sample preparation workflow. TD is capable of sampling a wide range of terpene concentrations by either adjusting the sample volume (how much air pulled through the tube during sampling) or by adjusting the split flow ratio of the thermal desorber prior to the analysis to transfer more or less of the sample to the GC instrument and detector.
Air sampling near live plants or dried plant materials provides a simple way to determine the concentration of the different terpenes released by the plant at a given time and under specific growing or drying conditions. With thermal desorption, only the volatile compounds like the terpenes are collected by the TD tubes. Less volatile compounds, such as tetrahydrocannabinol (THC), and cannabinoids, are not released into the air by the plant due to their much higher boiling points. So, for analysis only the TD tubes are sent to the laboratory, not any plant material. The technique is non-invasive/less destructive.
Knowing the terpene concentration emitted from the dried plant material could provide valuable information to growers and dispensaries, since the terpene odor can influence customer purchasing decisions. Below are examples of where Thermal Desorption tubes could be used for the determination/quantification of terpenes:
- Workplace Exposure – Industrial Hygiene
- to measure greenhouse worker’s exposure to terpenes.
- Emission & Odor Testing – Regulatory
- to quantify terpene emissions from a cultivation area (greenhouses or outdoor farms).
- Terpene Profiling – Growers & Testing Labs
- To characterize different strains
- Does a certain terpene ratio provide answers to questions like:
- When is the right time to harvest?
- When are the plants in distress?
- Testing terpene profiles can also be useful for other sources such as hops, fruits, and essential oils.
Thermal Desorption (TD) is a sample preparation technique used in the gas chromatographic analysis of volatile and semi-volatile organic compounds (VOCs and SVOCs).
Typically, an instrument called a Thermal Desorber is connected to a GC instrument and in most cases, it replaces the common GC inlet/injector port.
There are a variety of ways to collect samples onto TD tubes (active & passive). The most common way is to pull air through the TD tube using an air sampling pump. As the air travels through the tube the VOCs and SVOCs of interest are retained by one or more adsorbents packed into the tube while the main components of air (nitrogen, oxygen, argon etc.) passe through the adsorbents un-retained. Essentially the more volume of air that is pulled through the tube, the more VOCs and SVOCs are retained on the adsorbents in the TD tube (if no breakthrough occurs).
After sampling, the TD tube is sealed and sent to a laboratory where it is placed on a thermal desorber to be analyzed. During analysis the tube is rapidly heated to 250 – 330 °C while an inert carrier gas sweeps the VOC /SVOCs (thermally desorbed from the TD tubes adsorbent) onto the GC column, where they are separated and transferred to a detector such as flame ionization detector (FID) or a mass spectrometer (MS).
Experimental
To quantify the terpene concentration, a thermal desorption tube must be able to retain the terpenes during sampling, but it also must efficiently release them during the desorption step. During our research, we discovered no single adsorbent could provide good recoveries for all the terpenes relevant to cannabis and hemp samples. Therefore, we set out to develop a new multi-bed TD tube we named the Carbotrap® T420. It contains two different graphitized carbon black adsorbents that offer different surface areas and retentivity (weak and medium), resulting in excellent recoveries of the target terpenes (see Figure 1 for a schematic of the new tube). To demonstrate the performance of this new Carbotrap® T420 tube, we tested it alongside a TD tube packed with Tenax® TA, which is a commonly used adsorbent in the field of thermal desorption.

Figure 1.Schematic of the glass Carbotrap® T420 thermal desorption tube.
Terpene Test Standard
We combined two multi-component terpene test mixes and then added neat β-myrcene to the final mix to create a comprehensive terpene test mix for calibration and to challenge the TD tubes. See Table 1 for the specific details.
A final concentration of 400 µg/mL and 800 µg/mL was made by adding the following mixes to a 5 mL glass volumetric flask:
- 1 mL Terpene Mix A 2000 µg/mL (CRM40755)
- 1 mL Terpene Mix B 2000 µg/mL (CRM40937)
- 2.53 µL Myrcene neat (64643)*
- Fill flask to the 5 mL mark with methanol
*Volume calculated by using the density of myrcene 0.791 g/cm3 at 25°C
Analytical Conditions
For the analysis of the collected samples on the TD tubes the conditions and instrument parameters outlined in Table 2 & 3 were used.
The chromatogram in Figure 2 shows 0.5 µL of the terpene test standard (as described above) spiked onto a glass Carbotrap® T420 tube and then desorbed.
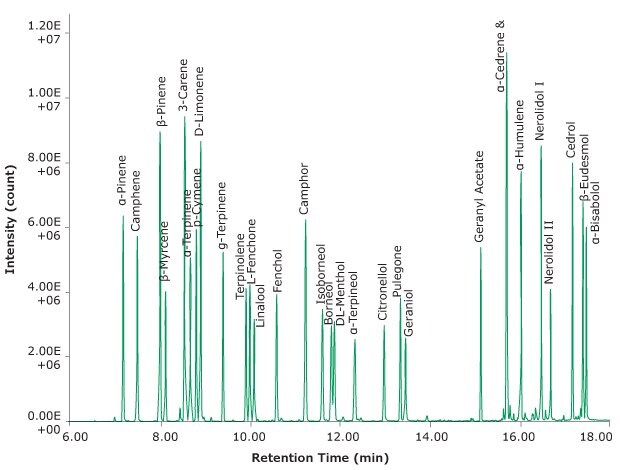
Figure 2.Example GC separation after desorption of the terpene test mix from the Carbotrap® T420 TD tube.
Results & Discussion
Recovery Experiments
To test the recovery, we spiked each of the TD tubes with 0.5 µL of the terpene test mix described above and challenged them with 2 and 10 liters of nitrogen gas. To spike the TD tube we used the Adsorbent Tube Injector System (ATIS). It is a sample preparation device designed to transfer calibration standards or test mixes onto a TD tube. ATIS employs the technique of flash vaporization to vaporize the sample in a continuous flow of inert gas, which carries the analytes to the tube. For this experiment, the ATIS flow controller was adjusted to deliver a constant flow of 0.1 L/min. (Figure 3 illustrates how the TD tubes were spiked). The temperature of the ATIS glassware was set to 100 °C. Using a Hamilton® 7000 series syringe, a 0.5 µL volume of the terpene test mix was injected into the ATIS glassware. The hot glassware vaporized the terpenes, which were then carried to the TD tubes by the nitrogen gas. The tubes remained attached until a total of 2 liters passed through the tubes. These steps were repeated with another TD tube, but the tube remained attached until a total of 10 liters had passed through the tube. After the tubes were challenged, they were analyzed to determine the recovery of each terpene desorbed from the TD tubes. To determine the recovery, a four-point calibration curve was created by spiking four Carbotrap® T420 tubes with 0.25, 0.35, 0.5, and 0.6 µL of the terpene test mix. For the calibration curve, the amount of nitrogen gas was reduced to a very small “challenge volume” of only 0.2 liters. This is enough to transfer the terpenes to the adsorbents packed in the tube but doesn’t present any sort of real challenge for the adsorbent since the volume is very low. Table 4 lists the results of this challenge experiment and compares the recoveries of those terpenes relevant to cannabis and hemp from a single-bed TD tube packed with Tenax® TA vs the new Carbotrap® T420 tube. Recoveries greater than 92% were achieved with the Carbotrap® T420 tubes at both 2 and 10 liters. Whereas the recovery on the Tenax® tube for α-pinene and camphene was less than 80% only after the 2-liter challenge and dropped to less than 25% together with β-pinene to 42% after being challenged with 10 liters.

Figure 3.Spiking the Carbotrap® T420 tube with the terpene test mix using Supelco® Adsorbent Tube Injector System (ATIS).
To compare the performance of the Carbotrap® T420 tubes under actual field conditions, we collected air samples from inside the trim room of a cannabis greenhouse using both the Carbotrap® T420 and Tenax® TA TD tubes. The air sample collection took place with both the TD tubes positioned side by side to at a flow rate of 0.1 L/min for a total sample volume of 10 Liters. Table 5 shows that the concentration obtained from the Tenax® tubes was significantly lower for α-pinene, camphene, and β-pinene. Typically, a difference of +/- 25% suggests that breakthrough (insufficient retention) could be occurring. This large difference suggests that with Tenax® TA an underestimation of the concentration for these more volatile terpenes can occur. This issue was not detected with the Carbotrap® T420. The two employed Graphitized Carbon Black (GCB) adsorbents in the Carbotrap® T420 with increasing adsorption strengths, make the tube more efficient and suitable for sampling a wider range of terpenes compared to tubes packed with just Tenax® TA
The lower recoveries of α-pinene, camphene, and β-pinene obtained from the Tenax® TA tubes from both the lab challenge and actual air samples taken in the cannabis-growing facility shows the importance of choosing a thermal desorption tube like the Carbotrap® T420 to produce accurate terpene air concentration values when collecting samples.
Uptake of Moisture During Air Sampling
Generally, it is important to minimize the amount of water vapor transferred to the GC during a thermal desorption process. As a rule of thumb, it is advisable to make sure that the amount of water retained on the TD tubes is always <1 mg prior to desorption. This can be achieved by choosing hydrophobic adsorbents (like used in the Carbotrap® T420) to reduce water pick up during sampling, and/or dry purging the tubes prior to the thermal desorption step. Since humidity inside a greenhouse can easily exceed 50%RH, it is important to keep this in mind when collecting air samples in this environment. Too much water vapor in the desorbed sample can affect split flow ratios as water vapor expands differently than dry helium (used carrier gas) since the water vapor is quickly released from the TD tube during desorption. Water vapor can also cause separation problems on the GC and quench some detectors. A commonly successful way to address this issue is to choose thermal desorption tubes that contain adsorbents that do not retain moisture during sampling. For this study, it was tested how much water was retained by the new Carbotrap® T420 TD tube.
For comparison, we also included TD tubes packed with Tenax® TA and silica gel. Tenax® TA was selected because it is known to be very hydrophobic. Silica gel was also tested. Due to its hydrophilic nature, it can retain up to ~40% of its own weight in water vapor before reaching saturation. Therefore, it would be indicative of the amount of water vapors being pulled through the other TD tubes during this experiment. For the experiment, we thermally conditioned the silica gel tubes at 120 °C and both the Carbotrap® T420 and Tenax® TA at 320 °C for one hour to assure they were completely dry before we obtained the tare weight of each tube using a laboratory balance. Then the tubes were connected to the exposure chamber where a dynamic atmosphere of 70% humidity was generated. We used an air sampling pump to pull the humidified air through the tubes at a flow rate of 0.1 L/min. After every 1-liter of humidified air pulled through the tubes, they were removed and weighed on a laboratory balance. This was repeated until a total of 10-liters of the humidified air had passed through the tubes. Table 6 shows the water retained by each type of tube. The results showed no significant amount of water vapor retained on the Carbotrap® T420 or the Tenax® TA tubes. The tare weight of the tubes fluctuated between +/- 1 mg which indicated that the water vapors passing through these tubes were not retained. As expected, the silica gel tube did retain a significant amount of water vapor and continued to increase in weight as more humid air was pulled through the tube. Only very little water vapor was retained by the Carbotrap® T420 TD tubes even after 120 mg of water vapor (10 L air sample) had passed through them, demonstrating it is on par with the Tenax® TA tube in terms of moisture retention. This shows that the Carbotrap® T420 TD tube can be used for effective sampling in high humidity environments. This simplifies the desorption, as no dry-purge step is required prior to analyzing the tubes.
Conclusion
The Carbotrap® T420 thermal desorption tube has been specifically designed for sampling terpenes in air. It exhibits excellent recoveries of terpenes relevant to the cannabis and hemp industry for sample volumes of up to 10 liters of air. This new adsorbent tube is also suitable for sampling in humid atmospheres like greenhouses as it does not retain water.
Thermal desorption can be considered as an eco- friendly sample collection and analysis technique since the used tubes, like the Carbotrap® T420, can be re- conditioned and reused multiple times. Furthermore no (hazardous) solvents are required in the desorption process, that would need to be sourced and later disposed after the analysis.
The Carbotrap® T420 is available as both glass and stainless-steel tubes. Glass tubes do have the advantage of allowing a visual observation of the integrity of the adsorbent packing with repeated use and are generally considered to be more inert. Stainless-steel tubes are more durable and will not break when sampling under harsher conditions in the field. Each TD tube contains a unique barcode for easy sample identification and tracking. The tubes are designed to function with any thermal desorption instrument that accepts ¼ in. O.D. x 3.5 in. long tubes (6.35 mm O.D. x 89 mm length).
References
To continue reading please sign in or create an account.
Don't Have An Account?