Determination of Total Polyphenol Content in Apples and Bananas by UV-VIS Spectrophotometry
Eddy Tan, Associate Senior Scientist
May May Lee, Principal Scientist
R&D APAC Lab
Overview of sections
Figure 1.Gallic Acid
INTRODUCTION
Polyphenols are naturally occurring compounds in plants and can be found in fruits and vegetables. These phenolic molecules are known to impart antioxidant effects so have garnered interest in the study of disease prevention as it is known to combat cell damage. As polyphenols are diverse with over 500 different chemical structures1, it is difficult to analyze them individually. Therefore, it is common practice to determine the total polyphenol content before assessing specific subgroups of polyphenols.
Here, we determine the total polyphenol content for two fruits – apple and banana. We have used gallic acid as a standard to represent polyphenols following AOAC SMPR2. Polyphenols will be extracted by methanol and derivatized following the Folin–Ciocalteu method3 before analysis with a UV spectrophotometer.
We reference our results to an established database for polyphenols - Phenol-Explorer database.4
The fruits were purchased from a local supermarket. Apples were from New Zealand and bananas were from Philippines.
EXPERIMENTAL
A. Gallic Acid Standard Preparation
Gallic acid standard stock solution (~1000 µg/mL):
- Weigh ~20±5 mg of gallic acid into a 20 mL volumetric flask
- Dissolve in ~6 mL of methanol
- Ensure all solids are dissolved before topping up to mark with methanol
- Mix well before use
B. Sample Preparation
One whole apple (fresh):
- Work quickly to remove the apple skin with a peeler and discard the skin
- Half the apple and remove the stem and core including the seeds
- Cut the apple into ~1 cm pieces and use food blender to reduce its size to <0.5 cm
- Transfer to a mortar and grind to a puree consistency
One whole banana (fresh):
- Peel off the banana skin and discard
- Chop the banana into smaller pieces ~0.5 cm
- Transfer to a mortar and grind to a consistent mash
C. Gallic Acid Standard Addition Curve Preparation
Spiking in apple puree/banana mash samples for 4 calibration points
- Weigh ~2 ±0.01 g of apple puree/banana mash each into four 50 mL centrifuge tube
- Record all sample weighing (e.g., for apple samples: A1 to A4, for banana samples: B1 to B4)
- Spike samples with the gallic acid standard following the table below to create 4 calibration points. Varying volumes of the standard are matched with methanol so that the final volume is 7000 µL
For reagent blank, use a separate 50 mL tube and add 7000 µL of methanol only
D. Extraction of Polyphenols
- Vortex or shake tubes from C for 10 seconds
- Sonicate samples in an ultrasonic bath for 10 minutes
- Centrifuge sample tubes for 2 minutes at 7000 g
- Carefully transfer the supernatant to a 15 mL centrifuge tube
- Repeat the above steps twice more with 7 mL of methanol as extracting solvent. Pool all supernatant into the same tube.
- Filter all supernatant through a high particulate syringe filter (HPF: Glass Fiber + Hydrophilic PTFE) 0.20 µm into a 25 mL volumetric flask
- Rinse the tube with methanol and filter it into the same 25 mL volumetric flask
- Top up to mark with methanol and mix well
E. Folin & Ciocalteu Method
Folin & Ciocalteu Reagent Preparation
(a) 7.5% w/v Sodium carbonate in water
- Weigh ~15 ±0.01 g of sodium carbonate into a 200 mL volumetric flask
- Add ~100 mL of water (DI) into the volumetric flask
- Dissolve the sodium carbonate completely by swirling or shaking
- Top-up to mark with water (DI) and mix well
(b) Folin & Ciocalteu’s diluted reagent
- Pipette 10 mL of Folin & Ciocalteu’s phenol reagent into a 100 mL amber volumetric flask
- Add ~60 mL of water (DI) to flask and mix
- Top-up to mark with water (DI) and mix well
(c) Derivatization Step
- Pipette 1 mL of sample from D into a 15 mL centrifuge tube wrapped with aluminum foil
- Pipette 1 mL of reagent blank from C into a 15 mL centrifuge tube wrapped with aluminum foil
- Pipette 4 mL of (a) into individually prepared tubes
- Mix by shaking for 10 seconds and then spin down the sample
- Pipette 5 mL of (b) into individually prepared tubes
- Mix by shaking for 10 seconds
- Incubate on an orbital shaker for 60 minutes at room temperature
- After incubation, filter the samples and reagent blank through a 13 mm hydrophilic PTFE 0.22 µm syringe filter into a new 15 mL centrifuge tube
(d) UV-VIS Spectrophotometric Measurement
- Set the UV-VIS Spectrophotometer to 765 nm for measurement
- Transfer the reagent blank into a 10 mm glass cuvette
- Tap the cuvette gently to remove air bubbles if any
- Wipe down cuvette sides with KimWipes and insert them into the sample chamber of the spectrophotometer
- Zero spectrophotometer with reagent blank
- Drain the reagent blank into a waste beaker
- Rinse the cuvette with a small volume of the sample before filling the cuvette
- Tap the cuvette, wipe the sides, and insert it into the spectrophotometer
- Measure and record the sample absorbance
RESULTS AND DISCUSSION
For the gallic acid standard addition, the below results were achieved for the apple and banana samples. Also, a set of 3 samples were anaylzed on different days.
Apple Sample
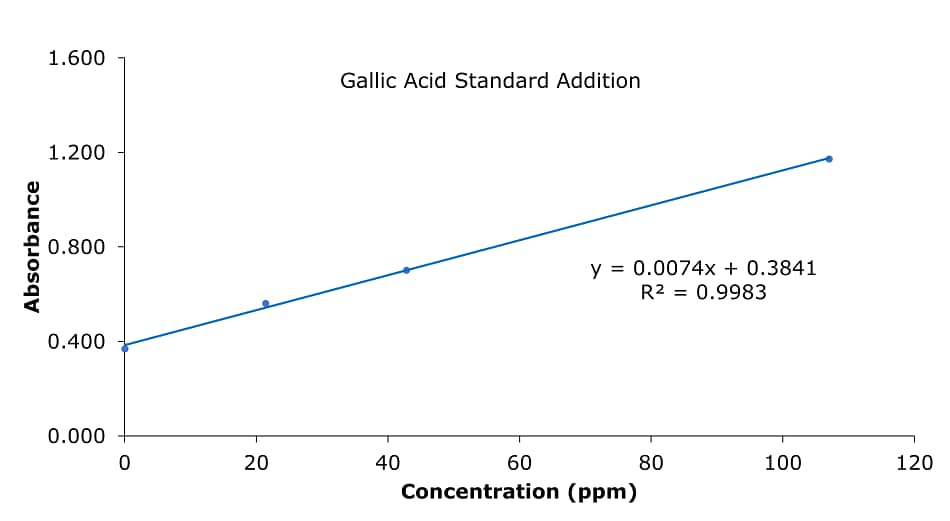
Figure 2.Gallic acid calibration curve (apple)
1. Standard addition plot
- y = 0.0074x + 0.3841,
When y = 0, x = | -0.3841 / 0.0074 |
= 51.91 µg/mL as gallic acid
2. Amount of gallic acid per 100 g of apple
- Average sample mass of 2.00 g extracted with 25 mL methanol (cf. three extraction steps 7 mL and make up to 25 mL). 1 ml extract derivatized to a final volume of 10 mL for absorbance reading. This dilution step (from Folin & Ciocalteu method derivatization) was not factored in the calibration data (Table 1), hence the sample concentration for calculation is 0.08 g apple per mL of the extraction solution.
Then 1 mL extraction solution has 0.0519 mg/mL gallic acid, or 0.08 g apple contains 0.0519 mg gallic acid. - 100 g apple has = (100 g x 0.0519 mg/mL)/0.08 g/mL = 64.88 mg polyphenols as gallic acid
Banana Sample
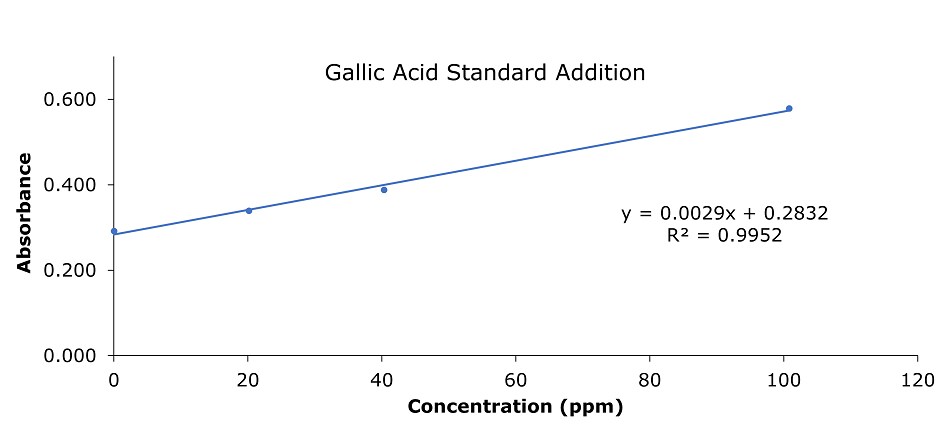
Figure 3.Gallic acid calibration curve (banana)
1. Standard addition plot
- y = 0.0029x + 0.2832
When y = 0, x = | -0.2832 / 0.0029 |
= 97.66µg/mL as gallic acid
2. Amount of gallic acid per 100 grams of banana
- Average sample mass of 2.00 g extracted with 25 mL methanol (cf. three extraction steps 7 mL and make up to 25 mL). 1 ml extract derivatized to a final volume of 10 mL for absorbance reading. This dilution step (from Folin & Ciocalteu method derivatization) was not factored in the calibration data (Table 3), hence the sample concentration for calculation is 0.08 g banana per mL of the extraction solution.
Then 1 mL extraction solution has 0.0977 mg/mL gallic acid, or 0.08 g banana contains 0.0977 mg gallic acid - 100 g banana has = (100 g x 0.0976 mg/mL)/ 0.08 g/mL = 122 mg polyphenols as gallic acid
The polyphenol content in apple and banana samples for 3 sets of measurements were determined and compared to the phenol explorer database5,6 as well as to peer-reviewed publications7,8. Results showed were in the common ranges stated in the literature (Table 5). The result variance from this experiments here is likely caused by the small batch sampling and also, by varying degrees of ripening as the tests were performed on different days.
CONCLUSION
We have shown that the polyphenol content in apples and bananas following the Folin & Ciocalteu method can be determined using the Spectroquant® Prove 600 (Prove 600 Plus) UV spectrophotometer. It was shown that the range of results from three sets of experimental data match the reported data in the phenol explorer database as well as peer-reviewed publications.
References
To continue reading please sign in or create an account.
Don't Have An Account?