HPLC Separation of Bile Acids with Ascentis Express C18
Clare Glicksman, Michael Wright, Dave Bell, and Anders Fridström
Reporter US, Volume 33.1
Helping to Elucidate the Role of Plasma Bile Acid Signalling in Humans
Introduction
Bile acids are increasingly recognized as playing an important signaling role in the control of immune response and energy metabolism. Recently, there has been interest in measuring bile acid concentrations in plasma and their impact on hormone concentrations, for example as affected by bariatric surgery (Figure 1).
These are two possible options for reducing dietary food intake. Both procedures create a small pouch at the start of the stomach by either restricting the stomach with a gastric band or by surgically creating a separate pouch from the stomach and then attaching it to the remainder of the GI tract.
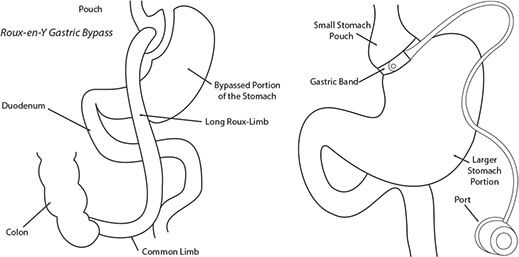
Figure 1.Roux-en-Y Gastric Bypass and Gastric Banding
Primary bile acids are synthesized in the liver from cholesterol. The majority are conjugated, increasing hydrophilicity, with either glycine or taurine. They are stored in the gall bladder and released into the small intestine on food consumption. Bacteria in the gut can convert primary bile acids to secondary bile acids which are then reabsorbed and taken back to the liver as part of enterohepatic circulation. Here secondary bile acids can also be glycine or taurine conjugated. Spill-over from this system results in bile acids in the peripheral circulation. There are potentially 15 species which can be measured in a peripheral plasma sample (Figure 2)
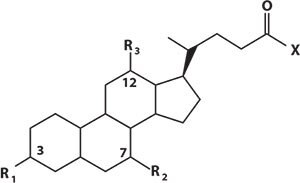
Figure 2.Structural Overview of Bile Acids and Conjugated Salts
Bile Acids and Conjugated Salts with Corresponding m/z Values
Methodology
The quantitation of plasma bile acids by tandem mass spectrometry MS/MS presents an analytical challenge. When employing collision-induced dissociation, bile acids will either form no useful fragments for quantitation (unconjugated), or produce a charged fragment originating from a conjugation group (conjugated taurine and glycine salts).
As three of the bile acids have the same molecular weight and elemental composition, they cannot be differentiated by MS/MS alone and require chromatographic separation of the group in both their unconjugated and conjugated forms. To enable separation, several orthogonal HPLC stationary phases were screened for selectivity. The best separation was achieved with an Ascentis® Express C18 analytical column based on Fused-Core® technology.
Sample Preparation
Plasma proteins were removed with addition of 900 µL of acetonitrile containing deuterated internal standards, to 250 µL of human EDTA plasma. The mixture was vortexed, centrifuged, and the supernatant evaporated before being reconstituted in a 50:50 solution of methanol and water. A 10 µL aliquot (corresponding to 8.57 µL of plasma) was injected into the HPLC.
Results and Conclusion
The resulting chromatogram showing the fifteen bile acid species separated on an Ascentis® Express C18 Fused-Core® column is shown in Figure 3. The method is fast and robust and allowed the compounds to be measured individually rather than the alternative measurement of a total concentration by colorimetric kinetic enzyme assays. This is particularly useful in research into the role of individual bile acids as signaling molecules. The method is suitable for clinical laboratories and is currently being used to investigate potential mechanisms linked to gut hormone profiles and glycemic control.
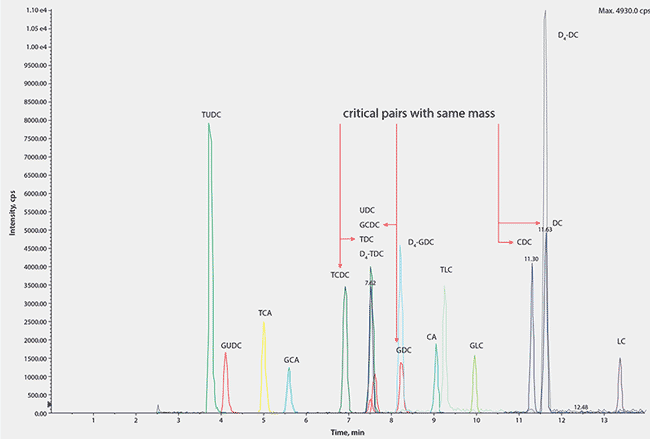
Figure 3.A Typical Chromatogram Showing all Analytes and Internal Standards with Full Resolution of all Derivatives with Similar m/z Values
Conditions
Column: Ascentis Express C18, 15 cm x 4.6 mm I.D., 2.7 µm (Product No. 53829-U); mobile phase: 5 mM ammonium acetate, 0.012% formic acid in [A] water; [B] methanol; gradient: 70 to 95% B in 10 min; held at 95% B for 1 min; flow rate: 0.6 mL/min; column temp.: 40 °C; detector: ESI(-), MRM mode (m/z shown in Figure 2); injection: 10 µL (corresponding to 8.57 µL of plasma); sample: protein-precipitated human plasma
Legal Information
Ascentis is a registered trademark of Sigma-Aldrich Co. LLC.
Fused-Core is a registered trademark of Advanced Materials Technology, Inc.
References
To continue reading please sign in or create an account.
Don't Have An Account?