All Photos(1)
About This Item
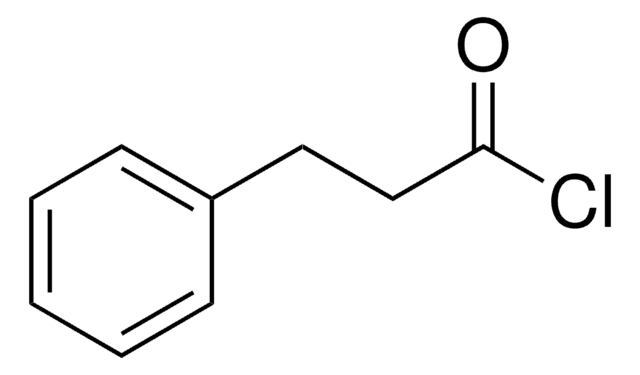
Linear Formula:
(C6H5)2CHCOCl
CAS Number:
Molecular Weight:
230.69
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
form
solid
bp
175-176 °C/17 mmHg (lit.)
mp
49-53 °C (lit.)
SMILES string
ClC(=O)C(c1ccccc1)c2ccccc2
InChI
1S/C14H11ClO/c15-14(16)13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,13H
InChI key
MSYLETHDEIJMAF-UHFFFAOYSA-N
Application
Diphenylacetyl chloride was used as a reagent in regioselective acylation of cyclomalto-oligosaccharides. It was also used in the preparation of N-substituted amides having local anesthetic properties.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
F El-Zahraa et al.
Die Pharmazie, 34(1), 12-13 (1979-01-01)
Diphenylacetyl chloride and pivaloyl chloride have been condensed with a wide variety of amines. Some of the resulting amides showed local anesthetic properties higher than those of procaine hydrochloride.
Jinyeon Hwang et al.
Experimental neurology, 335, 113511-113511 (2020-10-26)
Cyclin-dependent kinase 5 (Cdk5) is involved in neural organization and synaptic functions in developing and adult brains, yet its role in axonal regeneration is not known well. Here, we characterize Cdk5 function for axonal regeneration after peripheral nerve injury. Levels
F Santoyo-González et al.
Carbohydrate research, 262(2), 271-282 (1994-09-15)
Regioselective acylation of cyclomalto-oligosaccharides was achieved using pivaloyl and diphenylacetyl chlorides. The reaction of cyclomaltohexaose (1) with pivaloyl chloride gave the hexakis(2,6-di-O-pivaloyl) derivative 19 in 66% yield. Similar reaction with cyclomalto-heptaose (2) led to the octakis(2I,6I,6II,6III,6IV,6V,6VI ,6VII-O-pivaloyl) 26 and the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service