H36001
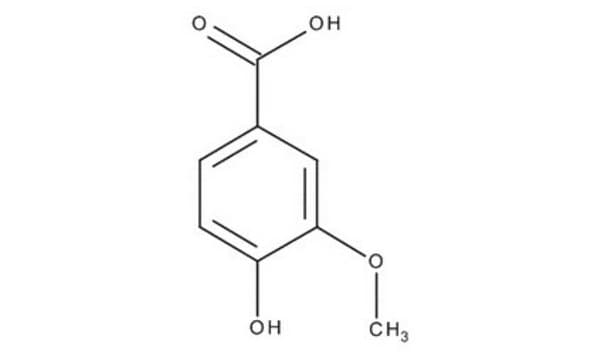
Vanillic acid
97%
Synonym(s):
4-Hydroxy-3-methoxybenzoic acid
About This Item
Recommended Products
Assay
97%
mp
208-210 °C (lit.)
SMILES string
COc1cc(ccc1O)C(O)=O
InChI
1S/C8H8O4/c1-12-7-4-5(8(10)11)2-3-6(7)9/h2-4,9H,1H3,(H,10,11)
InChI key
WKOLLVMJNQIZCI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Vanillin Synthesis from Vanillic Acid: Research focused on engineering the activity and thermostability of a carboxylic acid reductase for converting vanillic acid to vanillin, providing insights into biotechnological applications for flavor and fragrance industries (Ren et al., 2024).
- Antioxidant Properties in Food Preservation: The antioxidant properties of vanillic acid were evaluated in a study on the preservation of postharvest quality and physicochemical properties of broccoli, suggesting its potential in extending the shelf life and nutritional quality of fresh produce (Kibar et al., 2024).
- Biological Synthesis of Vanillin: A comprehensive review discussed various biological methods for synthesizing vanillin from vanillic acid, emphasizing its application in enhancing natural flavor profiles in the food sector (Venkataraman et al., 2024).
- aliphatic-aromatic polymers with good thermal stability and degradability
- novel polyesters via esterification and etherification reaction
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Protocol for HPLC Analysis of Flavonoids on Ascentis® RP-Amide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service