All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H14NO
CAS Number:
Molecular Weight:
152.21
MDL number:
UNSPSC Code:
12352000
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
90%
form
powder
reaction suitability
reagent type: oxidant
mp
182-189 °C (D)
storage temp.
2-8°C
SMILES string
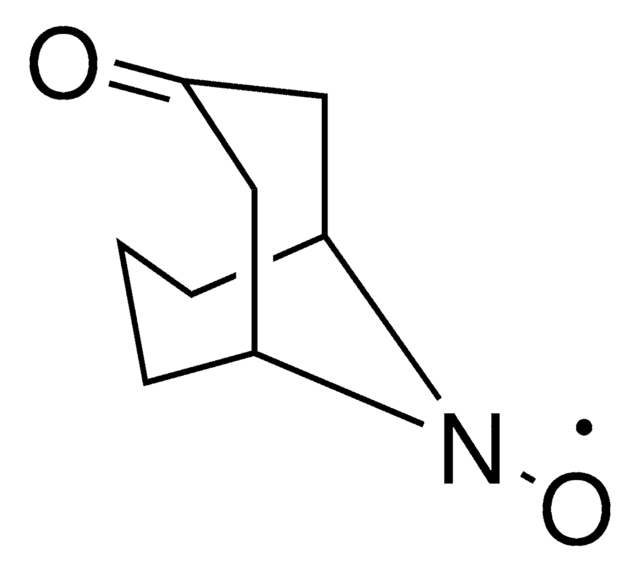
[O]N1[C@@H]2C[C@H]3C[C@@H](C2)C[C@@H]1C3
InChI
1S/C9H14NO/c11-10-8-2-6-1-7(4-8)5-9(10)3-6/h6-9H,1-5H2/t6-,7+,8-,9+
InChI key
BCJCJALHNXSXKE-SPJNRGJMSA-N
Related Categories
General description
2-Azaadamantane-N-oxyl (AZADO), a stable nitroxyl radical, is widely employed as catalyst for the oxidation of alcohols.
Application
2-Azaadamantane-N-oxyl (AZADO) may be employed in the following studies:
- As catalyst for the oxidation of wood cellulose.
- As catalyst in the total synthesis of Yaku′amide A, a potential cytotoxin obtained from sponge Ceratopsion sp.
- As oxidant for the oxidation of (S)-glycidol.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Takefumi Kuranaga et al.
Journal of the American Chemical Society, 135(14), 5467-5474 (2013-03-19)
Here we report the first total synthesis and the complete stereochemical assignment of yaku'amide A. Yaku'amide A (1) was isolated from a sponge Ceratopsion sp. as an extremely potent cytotoxin. Its structure was determined except for the C4-stereochemistry in the
Masatoshi Shibuya et al.
Journal of the American Chemical Society, 128(26), 8412-8413 (2006-06-29)
Development of a stable nitroxyl radical class of catalysts, 2-azaadamantane N-oxyl (AZADO) and 1-Me-AZADO, for highly efficient oxidation of alcohols is described. AZADO and 1-Me-AZADO exhibit superior catalytic proficiency to TEMPO, converting various sterically hindered alcohols to the corresponding carbonyl
Ming Zhang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(10), 3937-3941 (2015-01-22)
An increased supply of scarce or inaccessible natural products is essential for the development of more sophisticated pharmaceutical agents and biological tools, and thus the development of atom-economical, step-economical and scalable processes to access these natural products is in high
Takuya Isogai et al.
Biomacromolecules, 11(6), 1593-1599 (2010-05-18)
Curdlan, amylodextrin, and regenerated cellulose fiber were subjected to electromediated oxidation with a 4-acetamido-TEMPO catalyst in a buffer at pH 6.8 without NaClO or NaClO(2). More than 90% of the C6 primary hydroxyls of Curdlan and amylodextrin were converted to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)