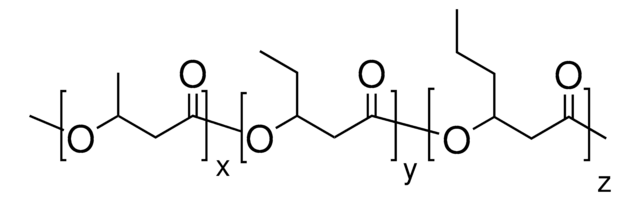
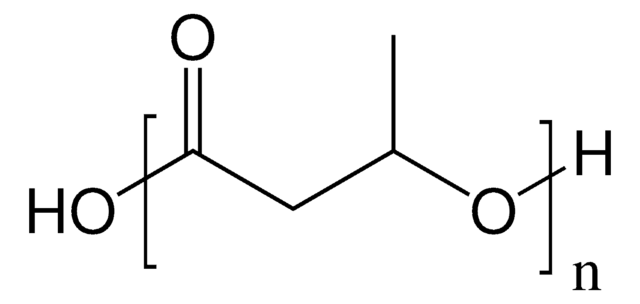
56655
(−)-Methyl (R)-3-hydroxyvalerate
≥98.0% (sum of enantiomers, GC)
Synonym(s):
(−)-Methyl (R)-3-hydroxypentanoate
About This Item
Recommended Products
Assay
≥98.0% (sum of enantiomers, GC)
optical activity
[α]20/D −37±3°, c = 1% in chloroform
refractive index
n20/D 1.427
bp
68-70 °C/5 mmHg (lit.)
density
1.029 g/mL at 20 °C (lit.)
SMILES string
CC[C@@H](O)CC(=O)OC
InChI
1S/C6H12O3/c1-3-5(7)4-6(8)9-2/h5,7H,3-4H2,1-2H3/t5-/m1/s1
InChI key
XHFXKKFVUDJSPJ-RXMQYKEDSA-N
Application
- Preparation of single-enantiomer 2-methyl-4-heptanol, a pheromone of Metamasius hemipterus, using (S)-2-methoxy-2-(1-naphthyl)propionic acid.: This research outlines the synthesis of a single-enantiomer pheromone compound using (S)-2-methoxy-2-(1-naphthyl)propionic acid. The process highlights the importance of enantiopure intermediates like (−)-Methyl (R)-3-hydroxyvalerate in the synthesis of biologically active compounds (Ichikawa and Ono, 2006).
Other Notes
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
168.8 °F - closed cup
Flash Point(C)
76 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

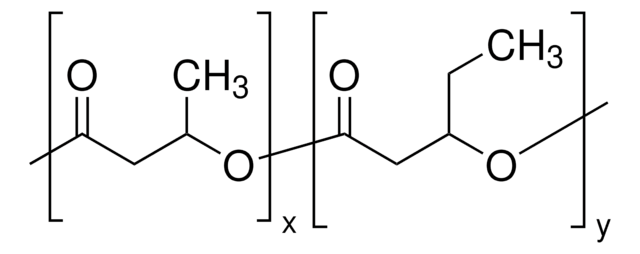
![Poly[(R)-3-hydroxybutyric acid] natural origin](/deepweb/assets/sigmaaldrich/product/structures/129/476/7d1c924b-f644-4889-a2d6-d7a923ce382c/640/7d1c924b-f644-4889-a2d6-d7a923ce382c.png)