484539
Germanium(II) sulfide
99.99% trace metals basis
Synonym(s):
Germanium monosulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
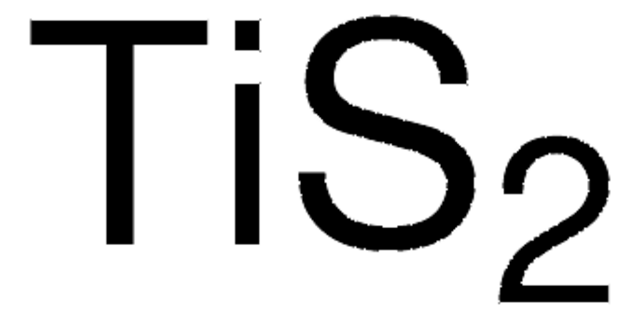
Linear Formula:
GeS
CAS Number:
Molecular Weight:
104.71
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
99.99% trace metals basis
reaction suitability
reagent type: catalyst
core: germanium
mp
615 °C (lit.)
SMILES string
S=[Ge]
InChI
1S/GeS/c1-2
InChI key
VDNSGQQAZRMTCI-UHFFFAOYSA-N
Related Categories
General description
Differential thermal analysis of germanium (II) sulfide was investigated. Melting point of GeS is 665oC. GeS nanoparticles may be prepared by gas phase laser photolysis, to be used in lithium ion batteries. GeS films may be generated by electrochemical deposition and used as an electrolyte. A study also reports the formation of GeS clusters by laser ablation.
Application
- New flexible and transparent solution-based germanium-sulfide polymeric materials: This research presents the successful preparation of solution-based polymeric germanium sulfide materials, showcasing their potential applications in flexible and transparent electronics (DTB De Salvi, AE Job, SJL Ribeiro, 2015).
- Germanium monosulfide as a natural platform for highly anisotropic THz polaritons: Introduces alpha-germanium(II) sulfide (GeS) as a promising candidate for enhancing THz nanospectroscopy, significantly impacting materials science and optoelectronic device development (T Nörenberg et al., 2022).
Packaging
Packaged in amber poly bottles
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sankaran Murugesan et al.
Langmuir : the ACS journal of surfaces and colloids, 28(13), 5513-5517 (2012-03-15)
A facile room-temperature electrochemical deposition process for germanium sulfide (GeS(x)) has been developed with the use of an ionic liquid as an electrolyte. The electrodeposition mechanism follows the induced codeposition of Ge and S precursors in ionic liquids generating GeS(x)
Yong Jae Cho et al.
Chemical communications (Cambridge, England), 49(41), 4661-4663 (2013-04-16)
Germanium sulfide (GeS and GeS2) nanoparticles were synthesized by novel gas-phase laser photolysis and subsequent thermal annealing. They showed excellent cycling performance for lithium ion batteries, with a maximum capacity of 1010 mA h g(-1) after 100 cycles. Metastable tetragonal
Joseph J Belbruno et al.
Physical chemistry chemical physics : PCCP, 12(30), 8557-8563 (2010-06-17)
Germanium sulfide clusters were generated by laser ablation of a solid sample. The resulting molecules were analyzed in a time-of-flight mass spectrometer. In addition to atomic germanium and diatomic sulfur, the spectra exhibited evidence for the existence of clusters containing
Thermal analysis of germanium (II) sulfide."
Ross L and Bourgon M
Canadian Journal of Chemistry, 46(14), 2464-2468 (1968)
Sean P Culver et al.
Journal of the American Chemical Society, 142(50), 21210-21219 (2020-12-08)
Strategies to enhance ionic conductivities in solid electrolytes typically focus on the effects of modifying their crystal structures or of tuning mobile-ion stoichiometries. A less-explored approach is to modulate the chemical bonding interactions within a material to promote fast lithium-ion
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service