C3172
Creatininase from microorganisms
lyophilized powder, 100-300 units/mg protein
Synonym(s):
Creatinine Amidohydrolase
Sign Into View Organizational & Contract Pricing
All Photos(6)
About This Item
Recommended Products
form
lyophilized powder
specific activity
100-300 units/mg protein
mol wt
~175 kDa
composition
Protein, 65-85%
foreign activity
Creatinase and urease ≤1%
Hexokinase and ATPase ≤0.1%
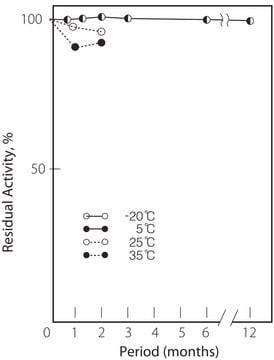
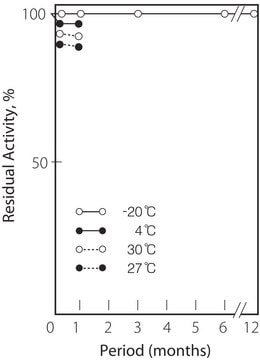
storage temp.
2-8°C
Application
Creatininase from microorganisms may be used in the preparation of amperometric biosensor by co-immobilization with other enzymes for the determination of creatinine.
This enzyme is useful for enzymatic determination of creatinine when coupled with creatine amidinohydrolase, sarcosine dehydrogenase or sarcosine oxidase and formaldehyde dehydrogenase in clinical analysis.
Biochem/physiol Actions
Creatininase from Pseudomonas sp. is a homohexameric enzyme with a molecular mass of 28.4 kDa per subunit. It is a cyclic amidohydrolase catalysing the reversible conversion of creatinine to creatine. Each monomer contains a binuclear zinc centre near the C termini of the β-strands and the N termini of the main α-helices. These zinc ions indicate the location of the active site.
Physical properties
Isoelectric point:4.7
Michaelis constants:3.2 x 10‾2M (Creatinine), 5.7 x 10‾2M (Creatine)
Structure:6 subunits per mol of enzyme (One mol of zinc is bound to each subunit)
Inhibitors:Ag+, Hg++, N-bromosuccinimide, EDTA
Optimum pH:6.5 − 7.5
Optimum temp:70°C
pH Stability:pH 7.5 − 9.0 (5°C, 16hr)
Thermal stability:Below 70°C (pH 7.5, 30 min)
Michaelis constants:3.2 x 10‾2M (Creatinine), 5.7 x 10‾2M (Creatine)
Structure:6 subunits per mol of enzyme (One mol of zinc is bound to each subunit)
Inhibitors:Ag+, Hg++, N-bromosuccinimide, EDTA
Optimum pH:6.5 − 7.5
Optimum temp:70°C
pH Stability:pH 7.5 − 9.0 (5°C, 16hr)
Thermal stability:Below 70°C (pH 7.5, 30 min)
Unit Definition
One unit will hydrolyze 1.0 μmole of creatinine to creatine per min at pH 8.0 and 25 °C
Physical form
Lyophilized powder containing sucrose and BSA as stabilizers
Analysis Note
Protein determined by biuret.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Antonino S Rubino et al.
The Annals of thoracic surgery, 91(2), 534-540 (2011-01-25)
Leukocyte filtration has been reported to reduce inflammatory damage during cardiopulmonary bypass. We evaluated the role of leukocyte filtration on hospital outcome and postoperative morbidity. Eighty-two consecutive patients who underwent isolated coronary artery bypass grafting were randomly assigned (1:1) to
Vannajan Sanghiran Lee et al.
Journal of computer-aided molecular design, 24(10), 879-886 (2010-08-31)
The reaction mechanism of creatinine-creatininase binding to form creatine as a final product has been investigated by using a combined ab initio quantum mechanical/molecular mechanical approach and classical molecular dynamics (MD) simulations. In MD simulations, an X-ray crystal structure of
Amperometric creatinine biosensor for hemodialysis patients
Tombach B, et al.
Clinica Chimica Acta; International Journal of Clinical Chemistry, 312(1-2), 129-134 (2001)
Kinuyo Yamashita et al.
Journal of molecular biology, 396(4), 1081-1096 (2010-01-02)
Creatininase is a binuclear zinc enzyme and catalyzes the reversible conversion of creatinine to creatine. It exhibits an open-closed conformational change upon substrate binding, and the differences in the conformations of Tyr121, Trp154, and the loop region containing Trp174 were
Tadashi Yoshimoto et al.
Journal of molecular biology, 337(2), 399-416 (2004-03-09)
Creatininase from Pseudomonas putida is a member of the urease-related amidohydrolase superfamily. The crystal structure of the Mn-activated enzyme has been solved by the single isomorphous replacement method at 1.8A resolution. The structures of the native creatininase and the Mn-activated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service