P78
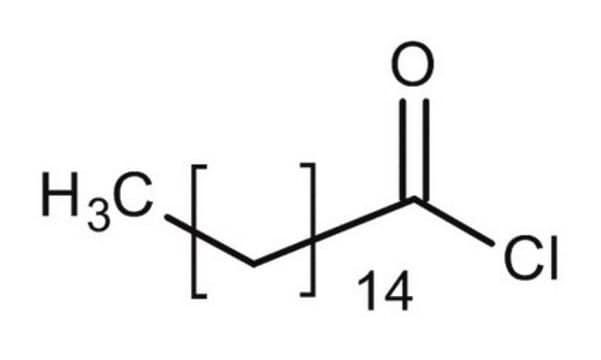
Palmitoyl chloride
98%
Synonym(s):
Hexadecanoyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)14COCl
CAS Number:
Molecular Weight:
274.87
Beilstein:
972409
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.452 (lit.)
bp
88-90 °C/0.2 mmHg (lit.)
mp
11-13 °C (lit.)
density
0.906 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCCCCC(Cl)=O
InChI
1S/C16H31ClO/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16(17)18/h2-15H2,1H3
InChI key
ARBOVOVUTSQWSS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Palmitoyl chloride can be used:
It can also be used in the total synthesis of:
- To introduce carbon chain in glycosphingolipid galactosyl ceramide through stereoselective olefin cross-metathesis.
- To prepare monoacyl and 1,3-symmetrical triacylglycerols via regioselective ring opening of an oxirane.
It can also be used in the total synthesis of:
- Hericenone J and 5′ -deoxohericenone C (hericene A).
- Seminolipid.
- Mycobactin S and T equivalents having catechol-glycine group instead of phenol-oxazoline of the naturally occurring mycobactins.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
320.0 °F - closed cup
Flash Point(C)
160 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Regioselective opening of an oxirane system with trifluoroacetic anhydride. A general method for the synthesis of 2-monoacyl-and 1, 3-symmetrical triacylglycerols
Stamatov SD and Stawinski J
Tetrahedron, 61(15), 3659-3669 (2005)
Rapid access to 6-bromo-5, 7-dihydroxyphthalide 5-methyl ether by a CuBr2-mediated multi-step reaction: concise total syntheses of hericenone J and 5′ -deoxohericenone C (hericene A)
Kobayashi S, et al.
Tetrahedron, 67(47), 9087-9092 (2011)
Takeshi Harayama et al.
Journal of lipid research, 56(7), 1370-1379 (2015-05-30)
The surfactant proteins (SPs), SP-B and SP-C, are important components of pulmonary surfactant involved in the reduction of alveolar surface tension. Quantification of SP-B and SP-C in surfactant drugs is informative for their quality control and the evaluation of their
Synthesis and studies of catechol-containing mycobactin S and T analogs
Walz AJ, et al.
Organic & Biomolecular Chemistry, 5(10), 1621-1628 (2007)
Total syntheses of seminolipid and its analogues by using 2, 6-bis (trifluoromethyl) phenylboronic acid as protective reagent
Shimada N, et al.
Organic & Biomolecular Chemistry, 17(31), 7325-7329 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service