All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H4O2
CAS Number:
Molecular Weight:
72.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
availability
not available in EU
refractive index
n20/D 1.426
density
1.124 g/mL at 25 °C
storage temp.
−20°C
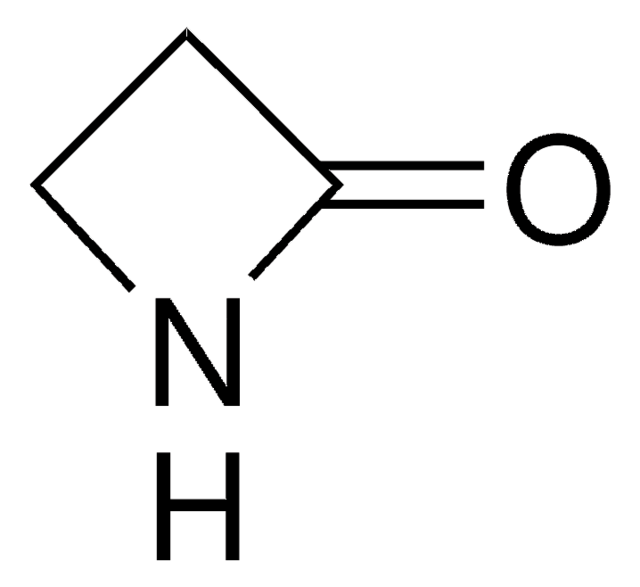
SMILES string
O=C1COC1
InChI
1S/C3H4O2/c4-3-1-5-2-3/h1-2H2
InChI key
ROADCYAOHVSOLQ-UHFFFAOYSA-N
Related Categories
General description
Product may polymerize over time during storage.
Application
3-Oxetanone can be used to synthesize:
- Various oxetane-containing lead compounds with improved solubility, reduced lipophilicity and amphiphilicity.
- (Hydroxymethyl)oxazoles and (hydroxymethyl)thiazoles via single-step microwave mediated reaction with primary amides and thioamides, respectively.
- Oxetane containing spirocycles through thermal 1,3-dipolar cycloaddition reaction with α-amino acids or secondary α-amino acid esters.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
79.0 °F - closed cup
Flash Point(C)
26.1 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Single-Step Microwave-Mediated Synthesis of Oxazoles and Thiazoles from 3-Oxetanone: A Synthetic and Computational Study
Orr D, et al.
Chemistry?A European Journal , 19(29), 9655-9662 (2013)
Synthesis of oxetane/azetidine containing spirocycles via the 1, 3-dipolar cycloaddition reaction
Jones B, et al.
Tetrahedron Letters, 57(25), 2811-2813 (2016)
Oxetanes as promising modules in drug discovery
Wuitschik G, et al.
Angewandte Chemie (International Edition in English), 45(46), 7736-7739 (2006)
Emily M Wright et al.
The journal of physical chemistry. A, 119(29), 7966-7972 (2015-06-25)
The pyrolysis products of gas-phase 3-oxetanone were identified via matrix-isolation Fourier transform infrared spectroscopy and photoionization mass spectrometry. Pyrolysis was conducted in a hyperthermal nozzle at temperatures from 100 to 1200 °C with the dissociation onset observed at ∼600 °C.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)