Fluticasone Propionate Assay by HPLC-UV following USP Monograph
Eddy Tan, R&D Scientist
R&D APAC Lab (Singapore)
Fluticasone propionate
Introduction
Fluticasone propionate is a glucocorticoid and is available as nasal sprays to treat sneezing, itchy or runny nose, or other symptoms caused by hay fever. It is also available as creams and ointments for topical applications to treat itchy skin.
The Purospher® STAR RP-18e Hibar® HPLC column, 250 x 4.6 mm, 5 µm, is used to demonstrate the system suitability criteria for the assay of fluticasone propionate following the USP monograph.
Experimental
Mobile Phase Preparation
- For buffer, weigh 1.15 g of monobasic ammonium phosphate in a 1 L volumetric flask and adjust with phosphoric acid to pH of 3.5 ± 0.05.
- For mobile phase, make up methanol, acetonitrile and buffer as 50:15:35 parts respectively.
System Suitability Solution, 0.05 mg/mL USP Fluticasone Propionate Resolution Mixture RS
- Weigh ~10 mg of USP Fluticasone Propionate Resolution Mixture RS into a 100 mL volumetric flask.
- Add ~90 mL of mobile phase and sonicate for 15 min.
- Top-up to mark with mobile phase and mix well. This is the System Suitability stock standard (~0.1 mg/mL).
- Dilute to a concentration of 0.05 mg/mL with mobile phase.
Fluticasone Propionate Solutions for Assay and Linearity Test
- Weigh ~10 mg of fluticasone propionate CRM into a 100 mL volumetric flask.
- Add ~90 mL of mobile phase to it and sonicate for 15 min.
- Top-up to mark with mobile phase and mix well. This is the fluticasone propionate stock standard (~0.1 mg/mL).
- Dilute to 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07 mg/mL with mobile phase in 5 mL volumetric flasks.
Fluticasone propionate solution for assay and relative standard deviation (RSD): 0.04 mg/mL.
Fluticasone propionate solutions for linearity, LOD and LOQ: 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07 mg/mL.
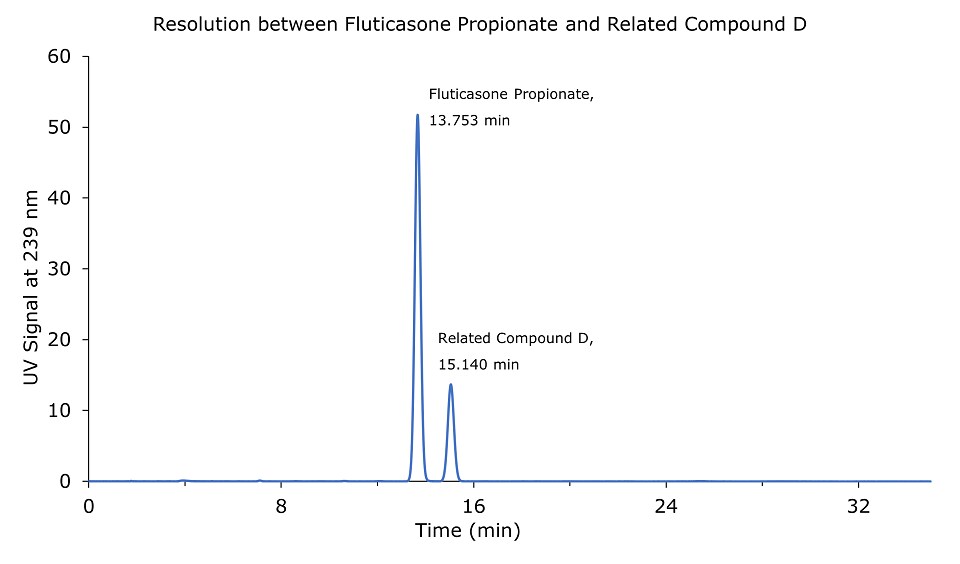
Figure 1.0.05 mg/mL USP Fluticasone propionate resolution mixture RS.
The relative retention times for fluticasone propionate and fluticasone propionate related compound D are 1.00 and 1.10 respectively.
Acceptance Criteria for System Suitability
Resolution: NLT 1.5 between fluticasone propionate and fluticasone propionate related compound D.
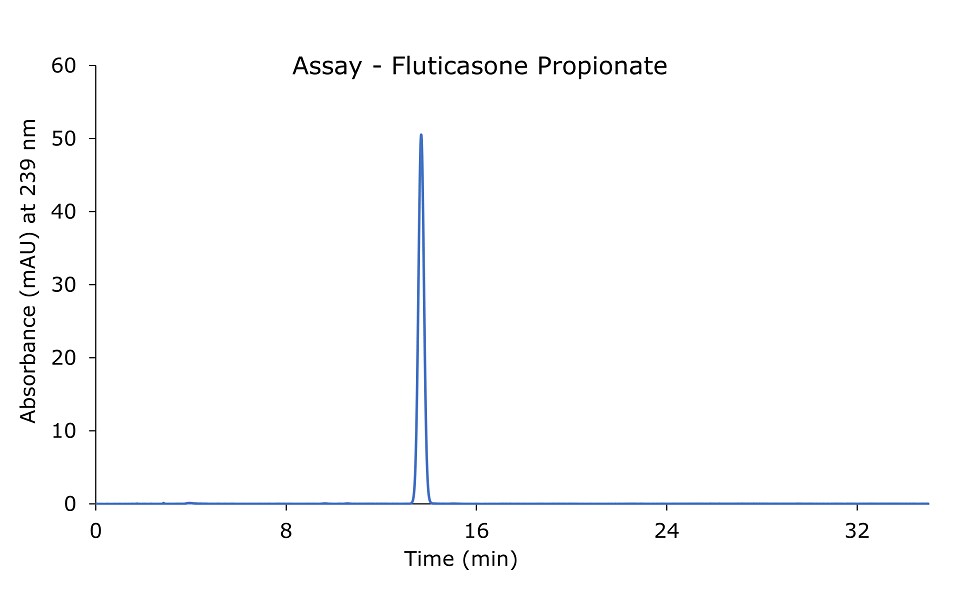
Figure 2.Fluticasone propionate 0.04 mg/mL standard solution.
Acceptance Criteria for Standard Solution
Relative standard deviation (RSD): NMT 2%
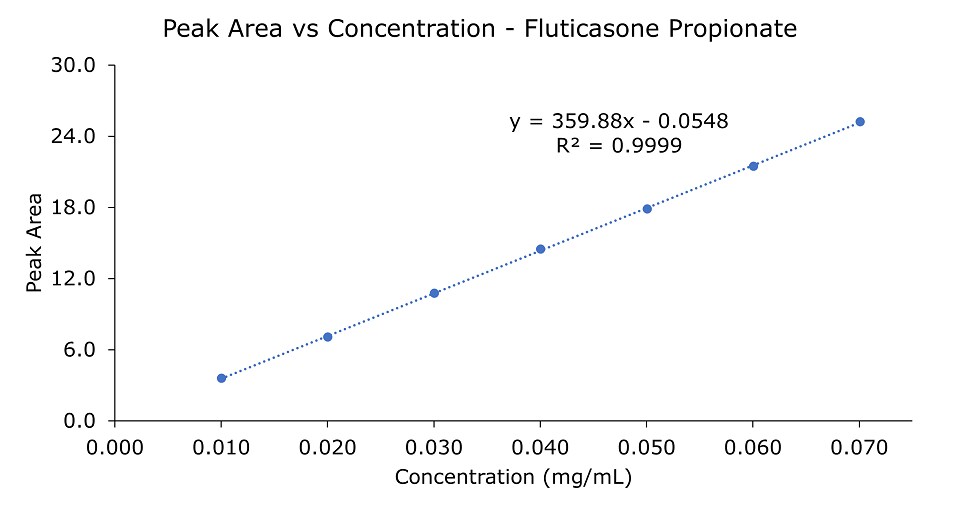
Figure 3.Linearity plot for fluticasone propionate solutions 0.01 to 0.07 mg/mL.
Conclusion
The Purospher® STAR RP-18e Hibar® RT column is able to comply with the system suitability criteria indicated in the USP monograph for the assay of fluticasone propionate. The resolution between fluticasone propionate and fluticasone propionate related compound D is greater than 1.5. The RSD of the standard solution is not more than 2%. Both criteria are within the assay specifications as defined in the monograph.
The column is robust with good reproducibility for repeated analyses (data not shown here). In addition, the column offers good linearity from 0.01 to 0.07 mg/mL fluticasone propionate with R2 of 0.9999. The Limit of Detection (LOD) for fluticasone propionate is 0.86 µg/mL and the Limit of Quantification (LOQ) is 2.6 µg/mL.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?