HPLC-UV Determination of Geniposide and Forsythin in Xiaoer Chiqiao Qingre Granules acc. to Ch. P. 2020 Method
Dean Duan, Senior Scientist
Merck R&D APAC Lab, Shanghai, China
Abstract
A Discovery® HS C18 HPLC column was used to analyze Xiaoer Chiqiao Qingre granules following the Chinese Pharmacopoeia 2020 monograph. The markers geniposide and forsythin (phillyrin) were analyzed from 1 to 100 and 1 to 200 µg/mL respectively. For the HPLC method, the LOD and LOQ for geniposide are 197.1 and 597.1 mg/g respectively, and for forsythin (phillyrin) 1.5 mg/g and 4.5 mg/g respectively.
Section Overview
Introduction
Xiaoer Chiqiao Qingre granules is a proprietary traditional Chinese medicine widely used to treat children with symptoms such as fever, cough, nasal congestion, sore throat, and constipation. There are 14 Chinese herbs in this preparation, all of which contain at least 6 active ingredients. Of the active ingredients, only geniposide and forsythin (phillyrin) are used as chemical markers in the Chinese Pharmacopeia 2020 monograph. Here, we use the Discovery® HS C18 column for the HPLC-UV analysis of geniposide and forsythin (phillyrin). The Discovery® HS column is specifically developed for pharmaceutical analysis and purification, showing no detectable bleed in the method and having good lot-to-lot reproducibility.

Experimental
Standard & Mobile Phase Preparation
Geniposide
Mobile phase
- Take 25 mL of isopropanol and add ~900 mL of water in a 1 L volumetric flask and mix well.
- Add 0.96 g of citric acid to the solution above and mix well. Top up to mark with water. This is a 5 mM citric acid aqueous solution containing 2.5% isopropanol.
- To 780 mL of the above, add 220 mL of methanol and mix well. This is the mobile phase for geniposide analysis.
Geniposide standards
- Weigh ~10 mg (accurate to 0.1 mg) of geniposide into a 10 mL brown volumetric flask.
- Add ~8 mL of the mobile phase above to it and sonicate for 5 minutes.
- Top-up to mark with mobile phase and mix well– this is 1 mg/mL of geniposide stock solution.
- Dilute the geniposide stock solution to 1, 2, 5, 10, 20, 50, and 100 µg/mL with the mobile phase.
Forsythin
Mobile phase
- Take 220 mL of acetonitrile into a 1 L volumetric flask. Top up with 1 L water and mix well. This is the mobile phase for forsythin analysis.
Forsythin standards
- Weigh separately ~10 mg (accurate to 0.1 mg) of forsythin (phillyrin) into a 10 mL brown volumetric flask.
- Add ~8 mL of mobile phase and sonicate for 5 minutes.
- Top-up to mark with mobile phase and mix well– this is 1 mg/mL of forsynthin stock solution.
- Dilute to 1, 2, 5, 10, 20, 50, 100, and 200 µg/mL with the mobile phase.
Sample Preparation
Geniposide determination
- Weigh 0.45 g of ground up Xiaoer Chiqiao Qingre granules into a conical flask.
- Add 25 mL of methanol to the flask and stopper tightly. Weigh flask and its content.
- Sonicate for 30 minutes at 300 W/50 kHz and cool to room temperature.
- Weigh again and use methanol to make up for the lost weight. Mix well and filter.
- Accurately take 2 mL of the filtrate into a 10 mL volumetric flask. Top up with mobile phase, and mix well for HPLC analysis according to the conditions in Table 2.
Forsythin determination
- Weigh 2 g of ground up Xiaoer Chiqiao Qingre granules into a conical flask. For a spiked sample for the determination of SPE recovery, add 1 mL of 200 µg/mL standard solution to 2 g of ground granules.
- Add 25 mL (24 mL for spiked sample) of methanol to the flask and stopper tightly. Weigh the flask and its contents.
- Sonicate for 30 minutes at 300 W/50 kHz and cool to room temperature.
- Weigh again and use methanol to make up for the lost weight. Mix well, and filter.
- Take 10 mL of the filtrate and evaporate to dryness.
- Add 5 mL of 70% ethanol to dissolve the residue.
- SPE clean-up of solution according to procedure in Table 1.
- Analyze according to the conditions in Table 3.
LC- Analysis
Prepared samples were analyzed by HPLC-UV according to the two compound specific methods described in Table 2 & 3.
Method Suitability
Acceptance Criteria: Theoretical plate number for geniposide and forsythin each: NLT 3000
Results
Chromatographic results of standards and samples are displayed in Figure 1–4. The calibration curves for geniposide and forsythin are displayed in Figures 5 and 6, respectively. The specificity of geniposide and forsythin standards are shown in Table 4. The specificity of geniposide and forsythin in Xiaoer Chiqiao Qingre granules is shown in Table 5. Repeatability data is in Table 6, and linearity is in Table 7. The SPE recovery for forsythin is shown in Table 8. The quantification results for the used Xiaoer Chiqiao Qingre granules are shown in Table 8.
See the conclusion for a summary of the results.
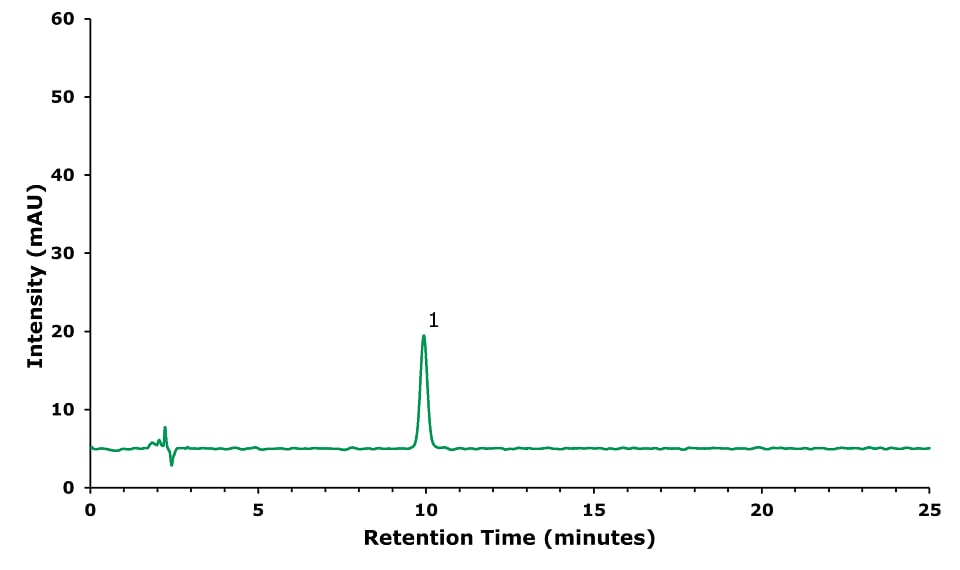
Figure 1.Geniposide (1) standard solution (20 μg/mL).

Figure 2.Geniposide (1) in Xiaoer Chiqiao Qingre sample solution.

Figure 3.Forsythin (1) standard solution (100 μg/mL).

Figure 4.Forsythin (1) in Xiaoer Chiqiao Qingre sample solution.
Chromatographic Data for Geniposide and Forsythin Analysis |
|---|
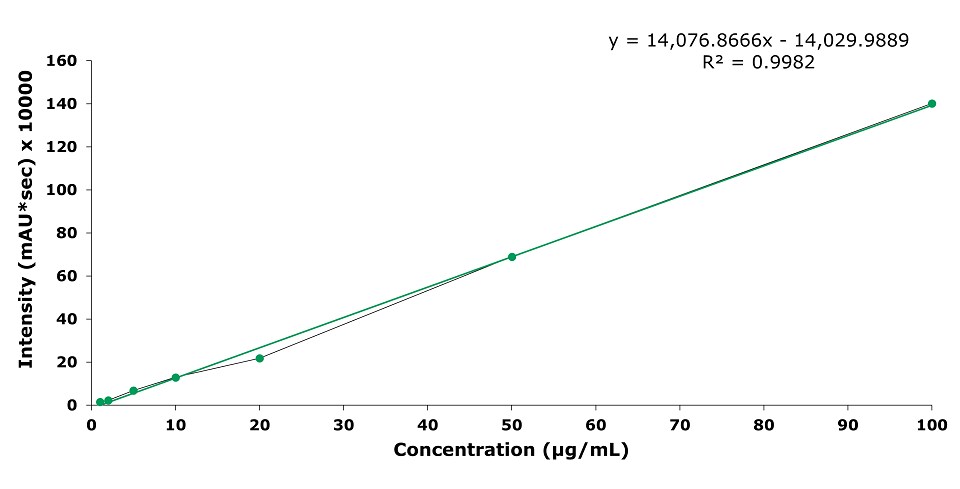
Figure 5.Calibration curve for standards of geniposide at 1, 2, 5, 10, 20, 50, and 100 µg/mL.

Figure 6.Calibration curve for standards of forsythin at 1, 2, 5, 10, 20,50, 100 and 200 µg/mL.
Conclusion
The Discovery® HS C18 column was used for the HPLC-UV determination of geniposide and forsythin (phillyrin) in Xiaoer Chiqiao Qingre granules according to the Chinese Pharmacopeia monograph. The acceptance criteria stated in the monograph were met by the two used methods. The R2 values for the linear range of both geniposide and forsythin calibration were >0.99.
The LOD and LOQ of geniposide were for the standards injections 0.89 and 2.69 µg/mL respectively, representing for the granule samples 197.1 and 597.1 µg/g respectively. The LOD and LOQ of the forsythin (phillyrin) standard were 1.5 and 4.5 µg/ml respectively, representing fro the granules 9.2 and 27.7 µg/g respectively. The Supelclean™ LC-Alumina-N SPE cleanup procedure for forsythin showed a recovery of 89.9% for forsythin.
We have shown that the Discovery® HS C18 column, together with Supelclean™ LC-Alumina-N SPE can meet the suitability criteria stated in the Chinese Pharmacopeia monograph for the analysis of Xiaoer Chiqiao Qingre granules.
See more applications on Pharmaceutical Analysis & Quality Control.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?