Strat-M® Membrane for Sunscreen Formulation Testing
Transdermal delivery is commonly used for delivering active skin care compounds. Development of effective sunscreens is a focus of skin care research, given that proper use of sunscreens can prevent occurrence of multiple skin ailments, such as melanomas, wrinkles and sunburns. For sunscreens to be useful in protecting against damaging ultraviolet (UV) rays, the active compound in the formulation should stay on the skin surface instead of penetrating the skin. Once a compound penetrates the skin, the compound can no longer exert its UV-protective effect.
Avobenzone and octocrylene are two commonly used sunscreen actives. Because there are always concerns about skin absorption of these compounds in their formulated forms, sunscreen developers typically perform in vitro transdermal diffusion studies using human skin as a model. In particular, octocrylene is known to penetrate into skin, wherein it acts as a photosensitizer, leading to DNA damage and malignant melanomas.
There are several barriers to using human skin for in vitro diffusion studies. Human skin is plagued with high lot-to-lot variability, and samples are expensive and not easy to obtain. Use of human tissue also poses experimental challenges, such as poor stability, sensitivity to storage conditions, biohazard concerns and disposal costs.

Figure 1.21 compounds, covering a range of molecular weight and lipophilicity, were tested for diffusion through human skin and Strat-M® membrane. The average cumulative flow (amount diffused per unit surface area over 8 hours) of each compound through human skin and through Strat-M® membrane were nearly equivalent, with a mean ratio of 1.61.
In this report, we show the utility of Strat-M® membrane in comparing transdermal diffusion of two sunscreen actives, avobenzone and octocrylene, in unencapsulated (Formulation A) and encapsulated (Formulations B and C) formulations. By encapsulation of the actives, diffusion of both the actives were substantially reduced. These results show that this membrane is a reliable and practical model for screening diffusion properties of sunscreen formulations.
Materials and Methods
Preparation of formulations. Oil-in-water emulsions were prepared using encapsulated and unencapsulated avobenzone and octocrylene. Formulation A consisted of unencapsulated 1% avobenzone and 3.5% octocrylene, whereas formulations B and C consisted of encapsulated avobenzone and octocrylene. Formulations A and B contained the same percentage of the active, the only difference being encapsulated vs unencapsulated version of the active. Formulation C contained encapsulated 2.1% avobenzone and 6.99% octocrylene. The rest of the formulations mainly consisted of water, isohexadecane and emulsion stabilizers.
Chromatographic analysis of actives. High performance liquid chromatography coupled with a diode array detector (HPLC-UV) was used for analysis and quantitation of avobenzone and octocrylene.
Human skin samples. Cadaver human skin samples were purchased from a skin bank. All experiments were conducted with three lots of human skin from three different donors. Individual experiments were conducted in triplicate and data were averaged for human skin from all the three skin lots as well as the membrane lot.
Vertical Franz cell diffusion studies. A 6-cell semiautomatic Franz cell setup was used for all diffusion experiments. Circulating water baths were set to 37 oC ± 0.5 oC. Strat-M® membrane (25 mm discs, Cat. No. SKBM02560) was placed between the donor and receptor compartments of the Franz cells. Fully hydrated cadaver skin was placed between donor and receptor compartments. The available open area between donor and receptor compartments measured 0.635 cm2. Compartments were clamped together, ensuring that the shiny side of the membrane was facing the donor compartment. The receptor compartment was filled with phosphate-buffered saline pH 7.4 containing 0.005% bovine serum albumin. When the receptor solution reached 37 oC, 500 μL of sunscreen formulation was added to the donor compartment. An aliquot of each sample (500 μL) was removed from the receptor compartment at times T=0, 1, 2, 3, 4, 5, 6, 8, 10, 24 and 26 hours.
Results
As expected, very low amounts of avobenzone and octocrylene were observed in the receptor fluid when formulation A was tested using the vertical Franz cell arrangement. Avobenzone and octocrylene showed very similar diffusion properties when compared between Strat-M® membrane and human cadaver skin (Figures 2 and 3). In the case of avobenzone, the cumulative amount diffused was between 20 and 70 ng/cm2, while in the case of octocrylene, the cumulative amount was between 5 and 20 μg/cm2. For both these compounds, the diffusion profile between human skin and Strat-M® membrane was very similar, indicating the utility of Strat-M® membrane as a screening tool for transdermal diffusion studies in the development of cosmetic products.

Figure 2.Diffusion of unencapsulated avobenzone through human skin and Strat-M® membrane (Formulation A).
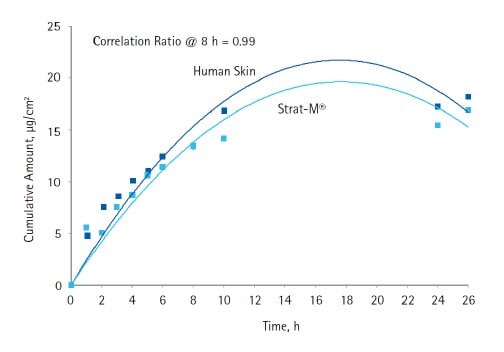
Figure 3.Diffusion of octocrylene through human skin and Strat-M® membrane (Formulation A).
In the case of Formulation A, the active ingredients were unencapsulated and in their native form. When the same actives were encapsulated and added to the sunscreen formulation, the amounts of both these actives diffusing through the membrane was significantly reduced. Diffusion of encapsulated avobenzone was approximately 10 times less than unencapsulated avobenzone (Figure 4). In fact, Formulation C, which contained twice as much avobenzone as in Formulation A, still showed a lower amount of diffused avobenzone compared to that observed with unencapsulated avobenzone (Formulation A).

Figure 4.Diffusion through Strat-M® synthetic membrane of avobenzone in unencapsulated (Formulation A) and encapsulated (Formulations B and C) formulations.
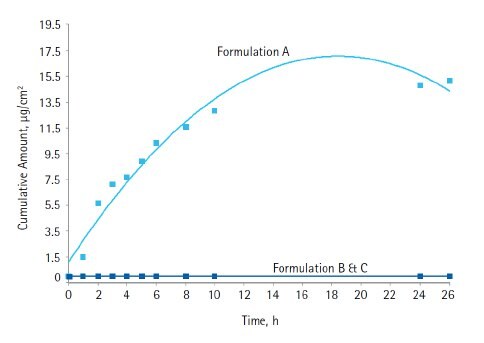
Figure 5.Diffusion through Strat-M® synthetic membrane of octocrylene unencapsulated (Formulation A) and encapsulated (Formulations B and C) formulations
Conclusion
Encapsulation of an active ingredient in a formulation can be crucial for modulating delivery of the active. In formulations, encapsulation can reduce the diffusion of sunscreen actives through skin, thereby increasing their screening activity as well as reducing potential toxic effects of diffusion.
We have described a synthetic membrane showing very close correlation to human skin when comparing diffusion of unencapsulated active. As has been shown in pig skin, encapsulation can decrease the transdermal diffusion of a sunscreen active by over three-fold [1]. Consistent with these observations, we determined that encapsulation of both avobenzone and octocrylene greatly decreased diffusion through synthetic membrane. For avobenzone, the reduction in diffusion by encapsulation was ten-fold, whereas for octocrylene, diffusion was reduced by almost 1000-fold.
We conclude that, for screening formulations for effectiveness of encapsulation on reducing transdermal diffusion of skin care actives, the use of a synthetic membrane model is a reliable, inexpensive, easy-to-handle alternative to human or animal skin.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?