Trichloroacetimidate Reagents
As described previously, trichloroacetimidates are also commonly employed as alcohol alkylation reagents, particularly when existing functionality is not acid sensitive.1 Recent applications of allyl trichloroacetimidate include the synthesis of an allyl propargyl ether intermediate in the synthesis of the fused-ring alkaloid securinine,2 preparation of fluorinated probes for protein kinase C (PKC),3 and in the formal synthesis of the oxocene target Laurencin (Scheme 1).4
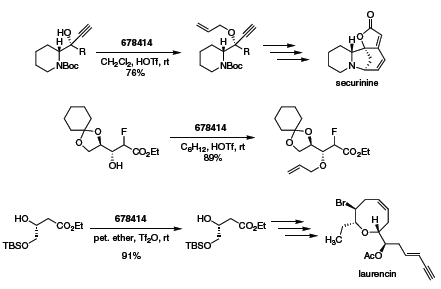
Scheme 1.( 678414 )
Likewise, 4-methoxybenzyl trichloroacetimidate has found extensive application for the protection of alcohols in the form of p-methoxybenzyl (PMB) ethers that are readily cleaved under oxidative conditions, typically dichlorodicyanoquinone (DDQ) or ceric ammonium nitrate (CAN). As shown in Scheme 2, 4-methoxybenzyl trichloroacetimidate was successfully applied in the selective preparation of chiral syn- or anti- diamines,5 as well as in independent syntheses of bryostatin intermediates (Scheme 3).6,7
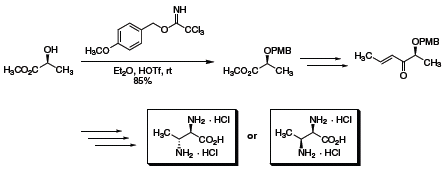
Scheme 2
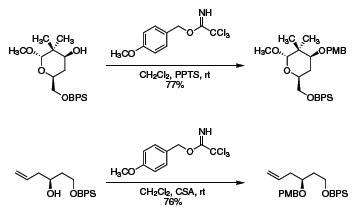
Scheme 3
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?