Homobenzotetramisole (HBTM): A General Organocatalyst for Asymmetric Acylations
Introduction
We are proud to offer the isothiourea organocatalyst homobenzotetramisole (HBTM) as part of our asymmetric catalysis portfolio in both (R) and (S) enantiomeric forms. These compounds have found broad applications in enantioselective transformations and kinetic resolutions of a variety of functional groups with low catalyst loadings.
Representative Transformations
Kinetic Resolutions
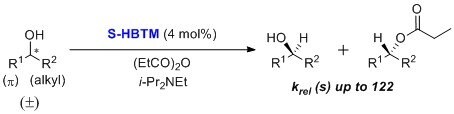
S-HBTM was originally developed by Birman and coworkers for use in kinetic resolutions of secondary alcohols as an alternative to enzymatic conditions.1 Selectivity factors of up to 122 are reported. The HBTM catalyst has been used in kinetic resolutions of allylic, propargylic, and benzylic secondary alcohols with a variety of organic solvents across a range of temperatures. Reactions are typically complete within a few hours. Optimal conditions were reported at lower temperatures (0 to –55 °C) in toluene, chloroform, or tert-amyl alcohol.1,2
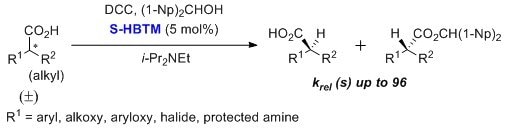
HBTM is useful in the kinetic resolution of a-arylalkanoic acids, a-aryloxy-alkanoic acids, α-alkoxy-alkanoic acids, α-haloalkanoic acids, and protected α-amino acids.3,4 Selectivity factors of up to 96 are reported. This transformation typically utilizes toluene as the solvent at low temperature over 24 hours.
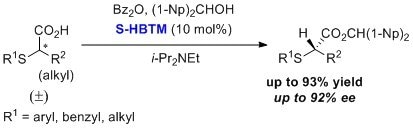
A dynamic kinetic resolution has been reported with HBTM for the formation of enantioenriched α-thioalkanoic acids.4,5 This process occurs with a broad substrate scope to produce high yields and high enantiopurities.
Enantioselective Transformations
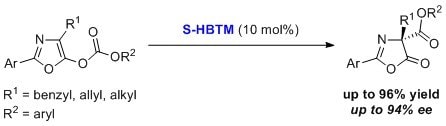
HBTM has been used for enantioselective carboxyl group transfer of oxazolyl carbonates.6 This process typically occurs in dichloromethane at low temperature for 16 hours to give high yields and high enantiopurities for a variety of substrates.
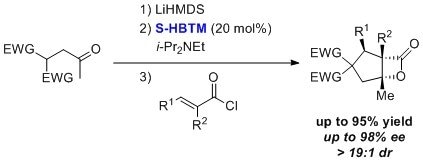
HBTM has been used for an enantioselective nucleophile-catalyzed, Michael-aldol-β-lactonization (NCMAL) sequence.7 This process occurs in tetrahydrofuran/dichloromethane over ≤ 24 h to give products with high diastereomeric ratios, high yields, and high enantiopurities.
Determination of Absolute Configuration
HBTM is the catalyst of choice for the Competing Enantioselective Conversion (CEC) method, which is used to assign the absolute configuration of enantioenriched stereocenters. The CEC method with HBTM has been reported for secondary alcohols, where reaction conversion is measured by 1H NMR8 and TLC.9 The TLC analysis has also been converted into an undergraduate laboratory experiment.10 This process occurs at room temperature, with the duration ranging from 30 min to a few hours.
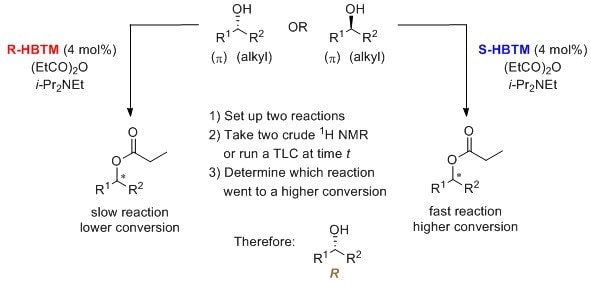
The following mnemonic is used to identify the absolute configuration of secondary alcohols using the R-HBTM and S-HBTM catalysts.8-10
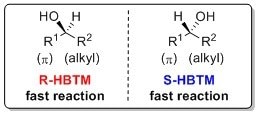
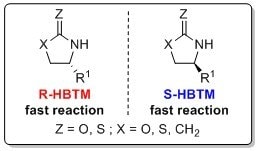
The CEC method with HBTM has also been reported to determine the absolute configuration of oxazolidinones, lactams, and thiolactams.11 This process is typically complete after a few hours at room temperature or 50 °C. The following mnemonic is used to identify the absolute configuration of these systems using the R-HBTM and S-HBTM catalysts.
Additional Studies
The nucleophilicity and Lewis basicity of HBTM have been studied based on rate and equilibrium constants from a series of experiments with benzhydrylium ions.12 Additionally, a detailed kinetic analysis of the HBTM-catalyzed esterification of secondary alcohols has also been conducted, which offers insight into the catalytic cycle for this transformation.13
Special thanks to Mr. Alex Wagner and Prof. Scott Rychnovsky for contributing this technology spotlight.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?