Nickel-based Catalysts
As a catalyst, nickel plays a central role in many synthetic transformations like oxidative addition, C-H activation, reductive elimination, oxidative cyclization, oligomerization, and carbon-carbon and carbon-heteroatom bond formation in cross-coupling reactions. Nickel is inexpensive compared to other transition metal catalysts; thus, it is used as an alternative catalyst for palladium in cross-coupling reactions.
Nickel is a transition metal that exists in the form of an alloy with aluminum or other metals, nickel salts, nickel supported on metal oxides; as Raney Nickel (porous form); or as an organometallic complex. Nickel complexes have a small nuclear radius, high paring energy, low electronegativity, and low redox potentials. They can adopt both high-spin and low-spin configurations to exhibit a range of stable oxidation states, as with nickel (0), nickel (II), nickel (III), and nickel (IV).1
What is a Raney Nickel Catalyst?
Raney nickel is a porous form of nickel along with a mixture of several other chemical elements. Nickel nanoparticles and Raney nickel are used as catalysts in the hydrogenation of carbonyls, aromatic compounds, and unsaturated hydrocarbons, and in desulfurization reactions.
Nickel Supported on Metal Oxides
Nickel alloys and nickel supported on different metal oxides, such as alumina and silica, are used as catalysts in the decomposition of methane to carbon dioxide and hydrogen, and in the hydrogenation of unsaturated fatty acids to saturated fatty acids.
Nickel Salts as Catalysts
Nickel chloride is used for nickel plating cast zinc, in electrolytic refining of nickel, and as a catalyst in diarylamine and silicon tetrachloride production. Nickel salts, like nickel chloride hexahydrate, is used as a catalyst in the synthesis of substituted pyrroles and piperidines.
Nickel Organometallic Complexes as Catalysts in Cross-Coupling Reactions
Organometallic catalysts consist of central metal surrounded by ligands. Nickel complexes, like Dibromo(1,2-dimethoxyethane)nickel(II), [1,2-Bis(diphenylphosphino)ethane]dichloronickel(II), and Bis(1,5-cyclooctadiene)nickel(0), are used as catalysts in oligomerization or polymerization of olefins, ring-opening reactions, and in cross-coupling reactions such as Mizoroki-Heck reaction (coupling of aryl halides and alkenes)2, Suzuki-Miyaura arylation (cross-coupling of aryl halides)3, Kumada coupling (asymmetric C-C cross-coupling), Heck reaction (C-C coupling between aryl halides or vinyl halides) and Negishi coupling (coupling of organozinc compounds with various halides (aryl, vinyl, benzyl, or allyl)).
The organometallic complex consists of a transition metal catalyst and ligands, e.g. phosphine ligands and BINAP. The ligands play a key role in stabilizing and activating the metal catalyst.
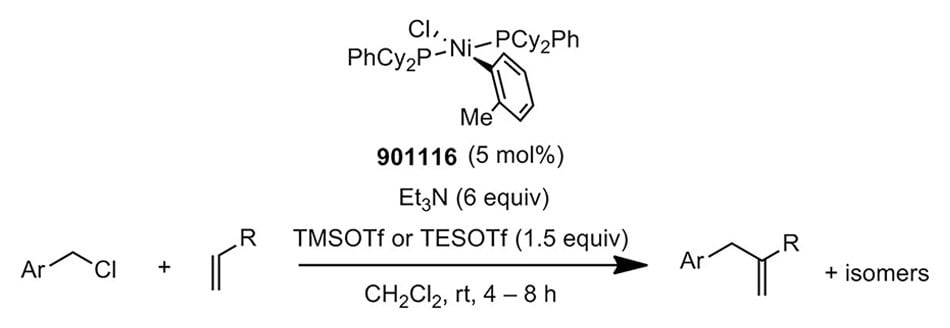
Representative Substrate Scope:
![bromo-benzo-b-thiophene 3-(5-Bromo-2-methylenepentyl)benzo[b]thiophene molecule](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/chemistry-and-synthesis/cross-coupling/bromo-benzo-b-thiophene/bromo-benzo-b-thiophene.jpg)
78% yield, 94:6
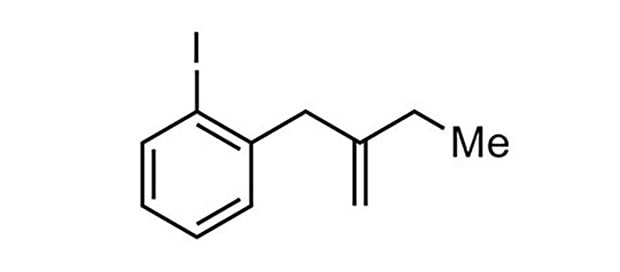
84% yield, 95:5

89% yield, 97:3
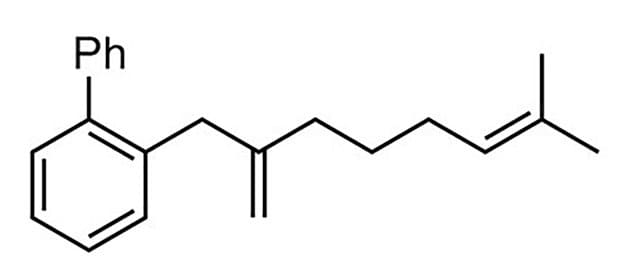
87% yield, 96:4
![fluoro-phenylmethyl 1-Fluoro-3-[2-(phenylmethyl)-2-propen-1-yl]benzene molecule](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/chemistry-and-synthesis/cross-coupling/fluoro-phenylmethyl/fluoro-phenylmethyl.jpg)
92% yield, 97:3
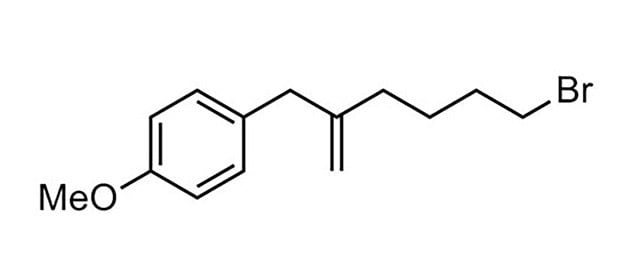
77% yield, 96:4
Ratios represent the major product to the sum of all other isomers.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?