FITC-labelled polysaccharides
FITC-Dextran
FITC-DEAE-dextran (FDD)
FITC-carboxymethyl-dextran (FCM-Dextran)
FITC-polysucrose (FITC-Ficoll®)
FITC-DEAE-polysucrose
FITC-CM-polysucrose (FITC-CM-Ficoll®)
FITC-inulin
Fluorescein hyaluronic acid (FHA-Se)
TRITC-labelled polysaccharides
TRITC-polysucrose (TRITC-Ficoll®)
Tetramethyl-rhodamine hyaluronic acid (TR-HA)
References
FITC-Dextran
Fluorescein Isothiocyanate Dextran
Chemical names:
Dextran(3´,6´dihydroxy-3-oxospiro(isobenzofuran-1(3H),9´-[9H]xanthen]-5(or 6)-yl)carbamothioate.
Fluorescein isothiocyanate-dextran.
Fluoresceinyl thiocarbamoyl-dextran.
CAS number: 60842-46-8
Structure

Definitions
Mw: Weight average mean molecular weight
Mn: Number average mean molecular weight
DS: Defines the number of substituent molecules per glycose molecule in the polysaccharide chain
Properties
Selected dextran fractions prepared from native Dextran B512F are labelled with fluorescein using a stable thiocarbamoyl linkage - the labelling procedure does not lead to any depolymerisation of the dextran1. The DS of FITC-dextrans ranges from 0.002-0.008 and at these low levels of substitution, confer minimal charges to the dextran; which is important for permeability studies.
FITC-dextran is supplied as a yellow/orange powder which dissolves freely in water or salt solutions giving a yellow solution. The product also dissolves in DMSO, formamide and certain other polar organic solvents but is insoluble in lower aliphatic alcohols, acetone, chloroform, dimethylformamide. High molecular weight fractions should be dissolved by slowly adding the powder to warm water (approx. 60 °C) with vigorous stirring.
The dextran molecule at molecular weights greater than 5,000 Daltons behaves as a flexible and extended coil in solution. Table 1 shows the molecular dimensions at various molecular weights. Dextrans and FITC-dextrans will exhibit Newtonian flow characteristics i.e. the viscosity is independent of shear rate.
Spectral Data
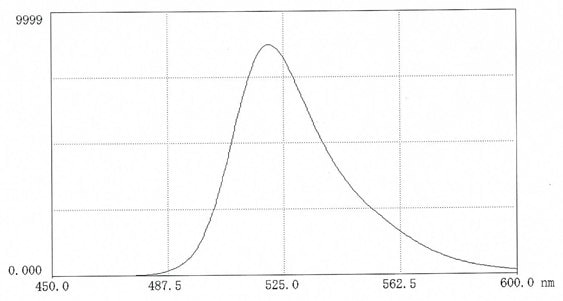
Figure 1.Fluorescence scan of FITC-dextran 70 in 0.025M borate pH 9.0 ( 10mg in 50 mL buffer). Excitation 495nm; Emission 520nm.

Figure 2.Fluorescence (Emission 520nm) of FITC-dextran in the range pH 4-9
Storage and Stability
The stability of FITC-dextrans in vitro and in vivo is excellent - only at elevated pH(>9) and elevated temperatures is there a risk for hydrolysis of the fluorescein label. Studies at 37 °C
in rabbit plasma, muscle homogenate, liver homogenate and urine established that FITC-dextrans are stable for at least 3 days2. No changes in the Mw. and no release of fluorescein moieties were noted. FITC-dextran is stable in 6% trichloracetic acid at room temperature for 3 days2. Autoclaved FITC-dextran 70 solutions were stored at temperatures from 8 to 50 °C for periods up to 5 months. Only samples stored at 50 °C showed a slight increase (1%) in free amino-fluorescein. FITC-dextran was found to be stable at pH 4 but at pH 9, a considerable (24%) decrease in fluorescence took place at 35 °C over 1 month. Several studies3 have confirmed the in vivo stability of FITC–dextrans during the experiments (1-6 days) .
Applications
FITC-dextrans are primarily used for studying permeability and transport in cells and tissues but may also be used for permeability studies of other materials (filters, gels etc). A particular benefit is that measurements of the fluorescence provide quantitative data on the permeability of healthy and diseased tissues in real time. Intravital fluorescence microscopy offers high sensitivity and concentrations down to 1μg/ml can be detected in tissue fluids. Several models have been developed for facilitating real time studies4,5 FITC-dextrans have also been use as a pH probe in cells6,7. It has also been noted from polarization experiments, that the lifetime of the excited state is similar to that before conjugation .
FITC-dextrans have proved valuable in the following fields of study (references selected from an extensive list on each topic):
- Permeability studies on intestinal tissues8-10
- Permeability studies of brain and nervous system11-13
- Permeability studies on neoplastic tissues14-16
- Permeability studies within the ocular chamber17-19
- Permeability studies of renal tissues20

Figure 3.Images taken from cheek pouch after infusion of FITC-dextran 150. The second image shows the leakage of the microvasculature after subjection to histamine. (photo permission of E.Svensjö).

Properties
FITC-DEAE-dextrans are supplied as a yellow powder which is freely soluble in water or electrolyte solutions. The rate of dissolution will depend on the particle size and structure and it is advisable to add the powder slowly with vigorous stirring – for high molecular weight products the water may be heated. The DS(FITC) lies between 0.001 - 0.008 and the limits for nitrogen content are 3-5% - corresponding to about one DEAE-substituent per three glucose units. The synthesis introduces two types of DEAE-substituents into the dextran chain(see structure above). The predominant substituent is the single tertiary group but this reacts further particularly at higher DS to yield a "tandem" group containing a quaternary amine as well as the tertiary amine.
Spectral Data

Figure. 4.Fluorescence scan of FITC-DEAE-dextran in 0.025M borate pH 9.0 ( 10mg in 50 mL buffer). Excitation 495nm; Emission 520nm.
Storage and Stability
The powder is stable at room temperature for more than three years providing it is stored in well-sealed containers in the dark. In solution, FITC-DEAE-dextran should not be stored at pH>8 for extended periods.
Applications
FITC-DEAE-dextrans will possess many of the properties shown by the parent DEAE-dextrans but will also display fluorescence to permit traceability. Some of the applications of DEAE-dextrans are given below:-
- As adjuvant for vaccines
- Enhances uptake of protein and nucleic acids by cells - transfection techniques and viral infectivity
- For stabilisation of proteins (enzymes)
FITC-DEAE-dextrans have been used to study delivery of positively charged molecules into nucleated cells via the perforin pore21. The permeability of FITC-DEAE-dextrans, FITC-dextran and FITC-dextran sulphate through nasal mucosa were compared in a study of absorption promoters22.
FITC-Carboxymethyl-Dextran (FCM-Dextran)
Chemical Names:
FITC-Carboxymethyl-dextran
Fluorescein-thiocarbamoyl-(O-carboxymethyl)-dextran
CAS number; not available
Structure

Properties
FITC-CM-dextrans are manufactured by reacting selected dextran fractions with an activated carboxymethyl derivative in alkali whereby O-carboxymethyl groups are introduced along the dextran chain. The carboxyl content is approximately 5% which is equivalent to about one CM group for every five glucose units. Thereafter, fluorescein groups are introduced by reaction with fluorescein isothiocyanate. The DS(FITC) lies between 0.003 - 0.008. FITC-CM-dextrans are supplied as a yellow powder which is freely soluble in water or electrolyte solutions. The products have a pronounced polyanionic character by virtue of the negatively charged carboxyl groups attached. The solution properties of FITC-CM-dextrans are expected to be comparable with those for CM-dextran23-25. In neutral solutions, the carboxymethyl substituents will repel each other leading to an expansion of the dextran coil. FITC-CM-dextrans are insoluble in most organic solvents, for example, ethanol, methanol, acetone, chloroform, ethyl acetate.
Spectral Data

Figure. 5.Fluorescence scan of FITC-CM-dextran in 0.025M borate pH 9.0 (10mg in 50 mL buffer). Excitation 493nm; Emission 519nm.
Storage and Stability
Prospective stability studies have established that CM-dextrans maintain their potency and purity for at least 3 years (unpublished studies) and from our experience with FITC-dextrans, we predict a similar stability for FITC-CM-dextrans. It is recommended that FITC-CM-dextrans are stored in air-tight containers in the dark at ambient temperatures.
Applications
CM-dextran itself has been found to be biocompatible and is used as a starting material in several pharmaceutical and diagnostic applications. The toxicity of FITC-CM-dextran is likewise anticipated also to be low. The insertion of a carboxyl group in the dextran chain provides further opportunities for immobilizing molecules with interesting biological activity(pharmaceuticals, enzymes, diagnostic tracers) on to dextrans.The carboxyl moiety may be used in many reactions, for example, esterification, amidation with amines, UGI or Passerini reactions. Simple ion-binding reactions can also provide a range of derivatives incorporating different cationic molecules26,27. The carboxyl groups will also impart an overall negative charge to the molecule, which may be valuable in gaining information on the permeability characteristics of cell membranes and tissues28. The application of FITC-CM-dextran in studies of drug delivery systems has been reported29.
FITC-Polysucrose (FITC-Ficoll®)
Chemical Names:
Polysucrose(3´,6´dihydroxy-3-oxospiro(isobenzofuran -1(3H),9´-[9H]xanthen]-5
(or 6)-yl)carbamothioate
Fluorescein isothiocyanate-polysucrose
CAS number; not available
Structure:

Properties
Polysucrose fractions are labelled with fluorescein by a procedure similar to that described by de Belder and Granath1. The fluorescein moiety is attached by a stable thiocarbamoyl-linkage and the labelling procedure does not lead to any depolymerisation of the polysucrose. The DS of FITC-polysucroses lies between 0.001- 0.008 and at these low levels of substitution confers minimal charges to the polysucrose.
FITC-polysucrose is supplied as a yellow powder which dissolves freely in water or salt solutions giving a yellow solution. The product also dissolves in DMSO, formamide and certain other polar organic solvents but is insoluble in lower aliphatic alcohols, acetone, chloroform, dimethylformamide.
The polysucrose molecule behaves as a globular molecule in solution as is to be expected from its structure. A comparison of the Stokes radius of dextran and polysucrose fractions reflects these differences in molecular flexibility (Table 2). The molecule is best regarded as an intermediate between a hard solid sphere and a flexible coil. Thus when comparing polysucrose and dextran fractions of similar molecular weights, the molecular dimensions of the polysucrose will always be smaller. Polysucrose solutions have very low osmotic pressures compared to sucrose solutions of equivalent concentration. Thus a 10% solution of polysucrose 70 has an osmolality of 3 mOs/kg compared to 150 for a 10% sucrose.
No detailed toxicity studies on FITC-polysucroses have been published. However, polysucrose fractions (100 000 to 500 000) when administered intravenously at doses up to 12g/kg in experimental animals showed no toxic symptoms. Polysucrose exhibits excellent biocompatibility with cells, virus, microorganisms etc. and has been used for many decades in cell separation technology.
Spectral Data

Figure 6.Fluorescence scan of FITC-polysucrose70 in 0.025M borate pH 9.0 (9.9mg in 50 mL buffer). Excitation 496nm; Emission 525nm. Measurements in biological media may significantly affect the fluorescence intensity which may be enhanced or depressed.
Storage and Stability
FITC-polysucrose powder when stored in air-tight containers at ambient temperatures is stable for at least 6 years. The stability of FITC-polysucroses in solution has not been investigated in detail. However, the stability of the thiocarbamoyl linkage between the fluorescein moiety and polysucrose will be similar to that with dextran (see FITC-dextran). FITC-dextran is stable at pH 4 for up to 1 month at temperatures up to 35 °C but this is not to be recommended for polysucrose based products owing to the lability of glycosidic linkages in sucrose. Polysucrose itself can be autoclaved at neutral and slightly alkaline pH.
Applications
The available data on the two polysaccharides, polysucrose (Ficoll®) and dextran, for assessing the glomerular permselectivity as compared to glomerular proteins has been reviewed30. Polydisperse polysaccharides are excellent probes for measuring glomerular permselectivity and are reproducible, reliable and elegant. The authors elaborate on the various properties which may influence the results such as molecular size, shape, charge and flexibility and assess their result in various pore models. Studies of the glomerular permeability of Ficoll when infused intravenously showed that it has a cut-off at about 50Å whereas dextran is excreted up to 60-70Å - this is explained by the greater flexibility of dextran. The clearance of FITC-polysucrose in mice lacking endothelial caveolae was studied in order to elucidate macromolecular transport pathways31. The glomerular filter was studied at different glomerular filtration rates using FITC-polysucrose 70 and 400 (also FITC-inulin)32.
Studies on the glomerular filtration of dextran and Ficoll showed that the glomerular membrane presented a much more restrictive barrier to Ficoll than to dextran33 . Interestingly, the values of the sieving coefficient for Ficoll approximated to those reported for uncharged globular proteins. Glomerular sieving in rats, following surgery and muscle trauma, was monitored using FITC-polysucrose 70/40034. The rats were dosed with a mixture of FITC-polysucrose 400 (960μg), FITC-polysucrose 70 (40μg) and FITC-inulin (500μg) as a priming bolus. Glomerular permeability was studied in caveolin-1 knockout mice using FITC-polysucrose 70/40035.
FITC-polysucrose 70 and albumin were infused in rats to explore the effects of temperature and ammonium chloride on the fractional clearance. Polysucrose performs differently from dextrans and was lower over the range 20-70 Å36,37. FITC-polysucrose 70 was used to evaluate whether the increase in clearance of native albumin after 9 weeks of diabetes was due to reduced charge selectivity or to an alteration in the proportion of large pores38.
FITC-DEAE-Polysucrose
Chemical Names:
FITC-(O-diethylaminoethyl)-polysucrose
Fluoresceinyl-thiocarbamoyl-(O-diethylaminoethyl)-polysucrose
CAS number; not available
Structure:

Properties
FITC- DEAE-polysucrose is supplied as a yellow powder which is readily soluble in water or buffer solutions and there is approximately one DEAE group for every five hexose groups. The limits for the degree of FITC substitution are 0.001 to 0.008. The product possesses a polycationic character. As depicted in the structural representation above, the DEAE-substituents may be present either as a single unit or as a ‘tandem’ unit – the latter containing a quaternary ammonium structure.
Spectral Data
Excitation is best performed at 493 nm and fluorescence measured at 523 nm (Figure 7). Measurements in biological media may significantly affect the fluorescence intensity which may be enhanced or depressed.

Figure 7.Fluorescence scan of FITC-DEAE-polysucrose 70 in 0.025M borate pH 9.0 ( 10mg in 50 mL buffer). Excitation 496nm; Emission 530nm. Measurements in biological media may significantly affect the fluorescence intensity which may be enhanced or depressed.
Storage and Stability
FITC-DEAE-polysucrose is delivered as a dry yellow powder and should be stored in well sealed containers at ambient temperatures in the dark. The pH of the product on delivery is 6.5 – 7.0 and should not be allowed fall below pH 5– the product should not be stored at pH > 7.0 for extended periods.
Applications
The product is used for studying the permeability of polycationic polymers relative to neutral polymers in organs, tissues and cells.
FITC-CM-Polysucrose (FITC-CM-Ficoll®)
Chemical Names:
FITC-(O-carboxymethyl)-polysucrose
Fluoresceinyl-thiocarbamoyl-(O-carboxymethyl)-polysucrose
CAS number; not available
Structure:

FITC-CM-Polysucrose Structure
Spectral Data
Excitation is best performed at 495 nm and fluorescence measured at 517 nm (Figure 8). Measurements in biological media may significantly affect the fluorescence intensity which may be enhanced or depressed. CHECK

Figure 8.Fluorescence scan of FITC-CM-polysucrose 70 in 0.025M borate pH 9.0 ( 11mg in 50 mLbuffer). Excitation 495nm; Emission 517nm.
Storage and Stability
FITC-CM-polysucrose is delivered as a dry yellow powder and should be stored in well sealed containers at ambient temperatures in the dark. Solutions may be kept at room temperature or preferably in a refridgerator in the dark at pH 6-7 for several weeks.
Applications
FITC-CM-polysucrose has played an interesting role in elucidating the properties of the glomerular membrane and it appears that despite the negative character of the membrane, the permselectivity of the anionic Ficoll derivatives is greater than the neutral species28. Later studies using FITC-CM-polysucrose, however, refuted these findings39. The significant role of solute charge in the sieving character of the glomerular membrane has been re-examined40.
FITC-Inulin
Chemical Names:
Inulin(3’,6’dihydroxy-3-oxospiro(isobenzofuran-1(3H) ,9’-[9H]xanthen]-5(or 6)-yl)carbamothioate
Fluorescein isothiocyanate-Inulin
CAS number; not available
Structure:

Properties
An inulin fraction obtained from dahlia tubers is labelled with fluorescein by a procedure similar to that described by de Belder and Granath1. The fluorescein moiety is attached by a stable thiocarbamoyl linkage and the labelling procedure does not lead to any depolymerisation of the inulin. The DS of FITC-inulin lies between 0.001-0.008 and at these low levels of substitution, the effect of the charges are minimal.
FITC-inulin is supplied as a yellow powder which dissolves in water or salt solutions giving a yellow solution. Dilute solutions (1-2%) remain clear on standing but more concentrated (>10%) may form precipitates on standing, since inulin tends to form crystalline aggregates. These precipitates will redissolve on heating. Temperatures up to 80 °C may be employed providing the solution is around neutral pH. The product also dissolves in DMSO, formamide and certain other polar organic solvents but is insoluble in lower aliphatic alcohols, acetone, chloroform, dimethylformamide, ethyl acetate.
The Mw of FITC-inulin as determined by SEC (Superose 6 + 12; dextran calibration) is approx. 5000. Phelps40 determined the Mw of inulin fractions from osmotic pressure data and obtained a value of 5640.
Spectral Data
Excitation is best performed at 490nm and fluorescence measured at 520 nm (Figure 9). Measurements in biological media may significantly affect the fluorescence intensity which may be enhanced or depressed. The dependence of fluorescence on pH is depicted in Figure 10.

Figure 9.Fluorescence scan of FITC-inulin in 0.025M borate pH 9.0 (10mg in 50 mL buffer). Excitation 492nm; Emission 519nm.

Storage and Stability
The stability of the thiocarbamoyl linkage between the fluorescein moiety and inulin will be similar to that for dextran (see data-file for information on stability of FITC-dextran). There are no prospective studies on the stability of FITC-inulin but retrospective studies have shown that FITC-inulin powder when stored in air-tight containers at ambient temperatures is stable for at least 6 years. Solutions of FITC-inulin should not be stored at pH(< 5) or high pH(> 9) for prolonged periods particularly at elevated temperatures.
Applications
FITC-inulin has been shown to be ideal for studying glomerular filtration rate in experimental animals as it is stable during filtration and renal passage and does not bind to plasma proteins or penetrate the renal cells. Measurements of the fluorescence provide quantitative data on transport and permeability of healthy and diseased tissues. Such studies can be performed in real time by intravital fluorescence microscopy. The technique offers high sensitivity and concentrations down to 1µg/mL can be detected in tissue fluids.
The tubular fluid to plasma concentration ratio was determined in rats following a bolus injection of FITC-inulin in the femoral vein41. The validity of the method as a measure of glomerular filtration was established by comparisons with 51Cr-EDTA and [H3]-inulin.
A thorough investigation of the use of FITC-inulin for GFR studies was presented by Fleck in 199942. The rats were given 4mg/mL at a rate of 4mL/100g bodyweight per hour via the tail vein or jugular vein. Dunn and coworkers43 found excellent correlation between creatinine clearance and FITC-inulin clearance in mice. Other studies using FITC-inulin for determining glomerular filtration have been described44,45.
FITC-inulin has been used for studying the permeability of intestinal epithelial cells46,47.
Fluorescein Hyaluronic Acid (FHA-Se)
Chemical Names:
5-aminofluorescein-labelled hyaluronate
5-aminofluorescein-labelled hyaluronan
CAS number; not available
Structure:

Properties
Hyaluronic acid, a polysaccharide composed of alternating β(1-3) glucuronide and β(1-4) glucosaminide units -derived from Streptococcus equi, is labelled with 5-amino-fluorescein giving a yellow fibrous product that is soluble in water and electrolytes, however, the solid requires prolonged gentle stirring – overnight – to dissolve48. The degree of substitution lies between 0.001 and 0.008. The molecular weight determined with a GPC system, calibrated with dextran standards, gave Mw of 6.0 x 106
Spectral Data
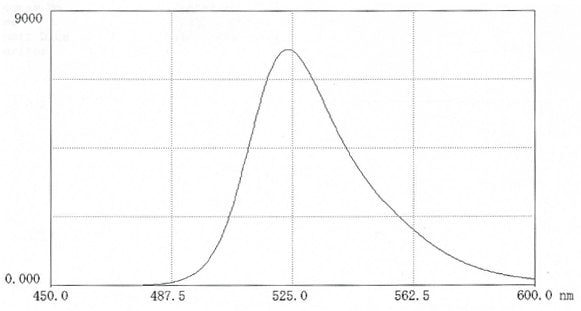
Figure 11.Fluorescence scan of FITC-hyaluronic acid in 0.025M borate pH 9.0 (12mg in 50 mL buffer). Excitation 495nm; Emission 524nm.
Storage and Stability
The dried product should be stored in air-tight containers at ambient temperatures in the dark. A shelf-life of 5 years is proposed. No release of fluorescent material was noted when a solution of the product was incubated at pH 7.5 at 37 °C for one month48.
Applications
Many applications of hyaluronan have appeared over the past years both in medicine(particularly its indispensable contribution to eye surgery) and in cosmetics. Fluorescein-labelled hyaluronic acid may be used as a probe for following the fate of hyaluronan in vitro. A FITC-labelled hyaluronic preparation greatly enhanced the visualisation of the permeation of the substrate through skin49. Other applications of fluorescein labelled hyaluronic acid have appeared50-53
TRITC-labelled Polysaccharides
TRITC-Dextran
Chemical names:
Tetramethyl-rhodamine isothiocyanate-dextran
Dextran 3´, 6´bis(tetramethylamino) -3-oxospiro(isobenzofuran-1(3H),9´-9H]xanthen]-5(or 6)- yl)carbamothioate.
Tetramethyl-rhodamine B thiocarbamoyl-dextran
CAS number; not available
Structure:

Properties
TRITC-dextrans are prepared from selected dextran fractions by coupling to tetramethylrhodamine B isothiocyanate (mixed isomers). The DS( TRITC) ranges from 0.001 to 0.008. At these low degrees of substitution, the charge contribution from the tertiary amino-groups on the rhodamine moiety is negligible. All batches are checked for molecular weight, loss on drying and free TRITC.
Spectral Data
Excitation is best performed at 550nm and fluorescence measured at 572 nm (Figure 12). Studies in our laboratories have shown that the fluorescence from a TRITC-dextran solution shows only slight changes over the range pH 3-9 (Figure 13). This is of interest when making quantitative measurements. Measurements in biological media may significantly affect the fluorescence intensity which may be enhanced or depressed.

Figure 12. Fluorescence scan of TRITC-dextran 40 in 0.025M borate pH 9.0 (10mg in 50 mL buffer) Excitation 550nm; Emission 572nm.

Figure 13.Dependence of fluorescence intensity(em. 572nm) of TRITC-dextran with pH.
Storage and Stability
The stability of TRITC-dextrans has not been investigated in detail but it is presumed to be similar to that of FITC-dextrans since both cases the substituents are linked via a thiocarbamoyl unit. Only at elevated pH (>9) and elevated temperatures is there a risk for hydrolysis of the thiocarbamoyl linkage (see FITC-dextran). TRITC-dextran solutions(pH 6-7) can be stored at room temperature in the dark for several weeks. The dry powder when stored in well-sealed containers in the dark has a shelf life of 5 years.
Applications
TRITC-dextrans are primarily used for studying permeability and transport in cells, vessels and tissues. Measurements of the fluorescence can be made quantitatively and in real time by intravital fluorescence microscopy. The technique offers high sensitivity and concentrations down to 1μg/mL can be detected in tissue fluids. A further important property is that TRITC-dextran does not bind to artery walls54,55.
The microvasculature of the hamster cheek pouch has proved to be a useful model for studying plasma leakage resulting from various inflammatory conditions. The cheek pouches are examined by intravital fluorescence microscopy using suitable filter (490/520nm) and images are captured with a digital camera (Figure 14). A 5% TRITC-dextran 150 solution in normal saline is administered i.v. (approx. 100mg/kg bodyweight)56-58. For more information on these techniques see Thorball3. TRITC-dextrans have been used extensively for permeability studies in tissues and cells and only a few references selected at random are given59-65.

Figure 14.TRITC-dextran 150 injected in a hamster cheek pounch 15min after histamine challenge. (by kind permission of E.Svensjö).

Properties
TRITC-polysucrose is a derivative of polysucrose – a polymer synthesized by cross-linking sucrose with epichlorohydrin. TRITC-polysucrose is prepared by reacting polysucrose with TRITC under similar conditions to those used for TRITC-dextrans. TRITC-polysucrose is readily soluble in water and salt solutions over a wide range of pH. Polysucrose is more sensitive to acid than dextran so that care must be taken when working at acid pH. It is supplied as a red powder which is freely soluble in water.
Spectral Data

Figure 15.Fluorescence scan of TRITC-polysucrose 70 in 0.025M borate pH 9.0 (11mg in 50 ml buffer) Excitation 522nm; Emission 552nm.
Storage and Stability
TRITC-polysucrose powder when stored in air-tight containers at ambient temperatures is stable for at least 6 years. The stability of TRITC-polysucroses in solution has not been investigated in detail. The stability of the thiocarbamoyl linkage between the tetramethylrhodamine moiety and polysucrose will be similar to that with dextran (TRITC-dextran). However, low pH storage is not recommended for polysucrose-based products owing to the lability of glycosidic linkages in sucrose. Polysucrose itself can be autoclaved at neutral and slightly alkaline pH.
Applications
TRITC-polysucrose has similar applications to those described for FITC-polysucrose but has certain advantages. As mentioned earlier, the fluorescence of tetramethylrhodamine is less dependent on pH than FITC-labels. Also the longer emission wavelength avoids background interference in experimental environments.
A TRITC-polysucrose 70 has been used in studies of the renal endothelial barrier66,67. Antibody response to thymus-independent antigens has been studies with the aid of TRITC-Ficolls68.
Tetramethyl-rhodamine Hyaluronic Acid (TR-HA)
Chemical Names:
Tetramethyl-rhodamine hyaluronic acid
Hyaluronan, 6´bis(tetramethylamino) -3-oxospiro(isobenzofuran-1(3H),9´-9H]xanthen]-5(or 6)- yl).
Tetramethyl-rhodamine B hyaluronan
CAS number; not available
Structure:

Properties
Hyaluronic acid, a polysaccharide composed of alternating β(1-3) glucuronide and β(1-4) glucosaminide units -derived from Streptococcus equi, is labelled with amino-tetramethylrhodamine giving a red product that is soluble in water and electrolytes. The DS lies between 0.001 and 0.008. The Mw determined with a GPC system, calibrated with dextran standards, gave a value of 6.0 x 106.
Spectral Data

Figure 16.Fluorescence scan of TR-HA in 0.025M borate pH 9.0 (12mg in 50 mL buffer) Excitation 552nm; Emission 576nm.
Applications
Tetramethyl-rhodamine hyaluronic acid(TR-HA) has similar applications to those described for fluorescein hyaluronic acid(see earlier section) but has certain advantages. As mentioned earlier, the fluorescence of tetramethylrhodamine is less dependent on pH than FITC-labels. Also the longer emission wavelength avoids interference from background images in experimental environments. Invasive growth into brain tissue employing TR-HA and 2-photon imaging has been described69.
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?