Sample Preparation for Mass Spectrometry
Mass Spectrometry
Mass spectrometry (MS) is a powerful technique for identifying and quantifying molecules within complex mixtures, in applications ranging from proteomics studies to environmental analysis. Successful mass spectrometry requires extremely careful sample preparation, to avoid contamination and decrease sample complexity. We support sample preparation for MS and LC-MS with Millex® syringe filters, membrane discs and filter holders to purify mobile phases, especially gel electrophoresis reagents, and ZipTip® pipette tips.
- Millex® syringe filters for mass spectrometry samples
- ZipTip® pipette tips
- Samplicity® filtration system
Millex® Syringe Filters for Mass Spectrometry
Obtain immaculate, particle-free samples for LC-MS with the peace of mind that you will have minimum interference from impurities introduced from your sample preparation device. MS-compatible hydrophilic polytetrafluoroethylene (PTFE) Millex® syringe filters have been shown to minimize extractable impurities in mass spectrometry.

Figure 1.Millex® syringe filters show fewer extractables in mass spectra than other brands. Minimize the appearance of extraneous peaks in mass spectra by filtering samples with Millex® syringe filters.
Choose the Right Filter to improve Mass Spectra
Membrane filtration of samples can improve sensitivity and reproducibility of mass spectra. Five easy ways to choose the right syringe filter for your mass spectrometry sample take into account sample volume, chemical compatibility, particle load, extractables, and analyte binding.
1. Choose the Right Size Filter
Filters are typically available in various diameters (4 mm -50 mm).
Advantages of Larger Filters:
- Fast filtration
- Low pressure required for filtration, minimal chance of bursting
Disadvantages of Larger Filters:
- Higher sample hold-up volume which can mean loss of precious sample volume
- Higher potential for inaccuracy due to the binding of analytes to the filter
- Higher amount of extractable impurities contaminating the sample
The ideal filter size balances the filtration performance with the risk of extractables and analyte binding, and depends on your sample volume. Use this table to choose the right filter size for your sample volume.
Sample Volume and Appropriate Filter Size
Lots of Samples?
When preparing a large number of samples, using syringe filters or ultrafilters which filter one sample at a time can be very time consuming. Instead, try a multiwell filter plate! Filter 96 or 384 samples at once into a collection plate, and analyze directly on a LC-MS system.
2. Choose a Chemically Compatible Filter
Using a filter where the membrane or the housing material is not compatible with the sample can lead to incomplete filtration, extraction of impurities into the sample, even complete membrane disintegration. The table below charts the chemical compatibility of common filter membranes and housing materials. Choose wisely.
Chemical Compatibility of Various Membranes and Housing Materials
(E = Excellent, G = Good, P = Poor, N = No Data)
Even Low Extractables Can Interfere with Mass Spectra
Even with the low level of extractables obtained with polypropylene (PP) filters, for example, minor contaminating peaks resulting from leachables can interfere with mass spectra. Compare spectra in Figure 2.

Figure 2.(Left): Extractable profile of polypropylene syringe filters (0.45 µm) using mass spectrometry. (Right): Extractable profile of hydrophilic filters (0.45 µm) using mass spectrometry.
Acetonitrile was filtered through the syringe filters and first mL of filtrate was collected. This filtrate was infused into API 2000 at a flow rate of 20 µl / min. for 15 min. Total ion current and av. Mass spectra were measured using ESI + mode and M/Z range of 100 – 1000.
3. Use Prefilters for High-Particle Load or Viscous Samples
Various solutions were filtered through membrane only and membrane + glass fiber pre-filter containing syringe filters until the filter clogged. Results were reported as average of three measurements. If single membrane filters get clogged before your sample can be passed through, use a syringe filter which contains a prefilter. The prefilter helps trap larger particulates, protecting the final membrane from fouling and allowing higher volumes of sample to be filtered before the filter clogs. With a multilayered filter, you can filter 4-6 times more volume than a membrane-only filter.

Figure 3.Various solutions were filtered through membrane only and membrane + glass fiber pre-filter containing syringe filters until the filter clogged. Results were reported as average of 3 measurements.
Prefilter Caveat
Before you go out and try a prefilter, though, remember that prefilters are usually made of glass fibers, which can lead to higher levels of extractables or analyte binding. In such cases it might be better to filter sample through a filter which contains an inert / low protein binding filter and a prefilter.
4. Choose a Filter with Low Analyte Binding
Depending on the chemical nature of your analyte, the membrane in your syringe filter might nonspecifically bind the analyte, resulting in inaccurate and irreproducible results especially for low concentration samples. Glass fiber filters, especially, tend to bind strongly to proteins, peptides, and oligonucleotides. The figure below shows that nylon membranes show very high protein binding (~ 225 µg/cm2) whereas polyvinylidene fluoride (PVDF) show very low protein binding (~ 15 µg/cm2).
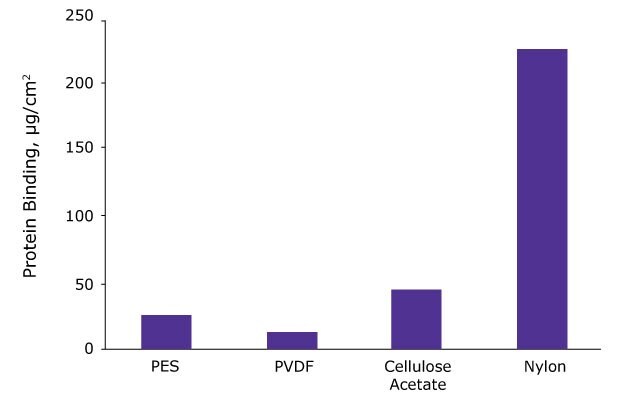
Figure 4.A comparative study of protein binding was done on various 0.2 µm membranes. 1 mg/ mL solution of 125I-labeled IgG was offered to 13 mm membrane discs. After incubation, bound protein was determined.
5. Rinse Filter with Solvent to Minimize Extractable Contamination
Even though extractables can pose problems in downstream analysis, these can be reduced significantly by just washing the membrane filter with the sample or a solvent prior to filtration. The figure below shows the effect of prerinsing of the filter on extractables. Just by rinsing the membrane with one mL of solvent, the extractables were reduced to undetectable levels by LC-UV.
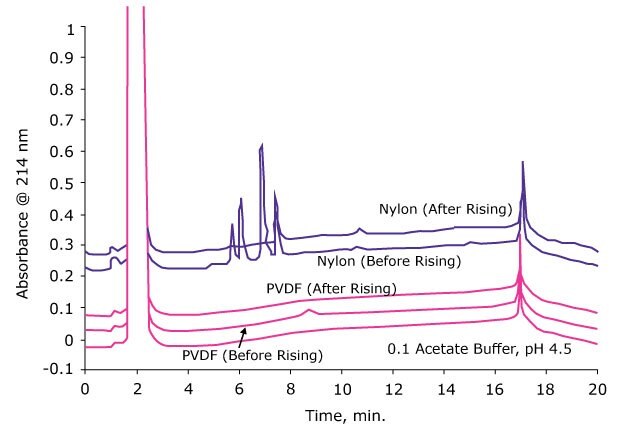
Figure 5.Various syringe filters were rinsed with 1.0 mL of extracting solvent and the second mL of filtrate was collected and analyzed using reversed phase HPLC.
ZipTip® Pipette Tips
A staple of every mass spectrometry lab, ZipTip® is a 10 μL pipette tip with a 0.6 or 0.2 μL bed of chromatography media fixed at its end with no dead volume. It is ideal for concentrating and purifying peptides or proteins in seconds prior to mass spectrometry, HPLC, and capillary electrophoresis. The ZipTip® pipette tip provides a reproducible, high recovery method for concentrating, purifying or even fractionating femtomoles to picomoles of peptides, proteins and oligonucleotides for improved data quality.
ZipTip® Advantages
- Single-step desalting, concentration, and purification
- Fractionate complex samples for more meaningful data
- Ideal for peptides, proteins, nucleic acids, and more
- No dead volume for maximum recovery
- Eliminates time-consuming chromatography
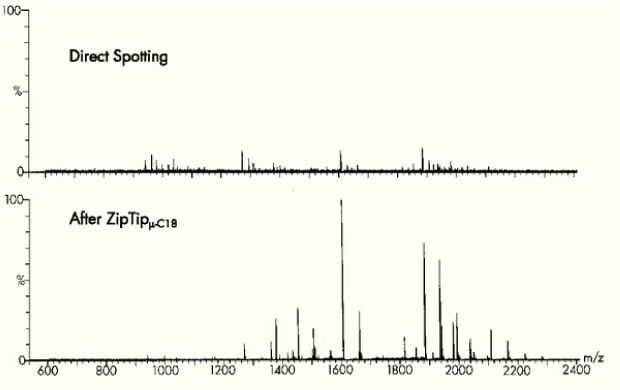
Figure 6.Mass spectrometry before and after preparation using ZipTip® pipette tips.
ZipTip® Pipette Tips
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?