LC/MS Analysis of Antiarrhythmic Drugs and Metabolites on Ascentis® Express HILIC
Materiales
analytical column
SPE tube or plate
standard
Amiodarone hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®N-Desethylamiodarone hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®(±)-Flecainide solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Lidocaine solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®CONDITIONS
column
Ascentis Express HILIC, 10 cm x 2.1 mm I.D., 2.7 μm particles (53939-U)
mobile phase
[A] 5 mM ammonium formate; [B] 5 mM ammonium formate in acetonitrile, 5:95 (A:B), adjusted to pH 7.0 with formic acid
flow rate
0.4 mL/min
pressure
1305 psi (90 bar)
column temp.
35 °C
detector
ESI+, 100-1000 m/z
injection
0.5 μL
sample
each compound, 300 ng/mL in 75:25 (1% formic acid acetonitrile:water)
Descripción
Analysis Note
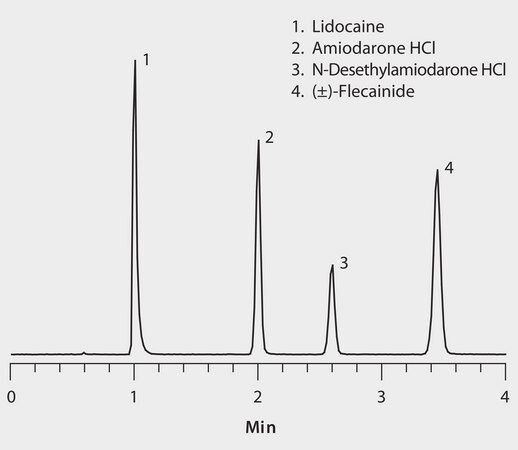
The basic nature of these antiarrhythmic agents makes them ideal candidates for HILIC (hydrophilic interaction) chromatographic separation. The benefit of HILIC over traditional reversed-phase chromatography is two-fold for both sample introduction and analyte detection. First, the high acetonitrile concentration of HILIC mobile phases allows for direct analysis of precipitated plasma samples without the need for additional sample solvent exchange. Second, the high acetonitrile content provides increased analyte response in positive ESI MS detection. Of the various HILIC-mode columns tested, method development for this assay determined the Ascentis Express HILIC, 2.7 μm particles, provided the best chromatographic resolution of the antiarrhythmic drugs while maintaining high peak efficiency for enhanced detection levels.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany