How Low-in-Nanoparticulate-Impurities Sucrose Can Enable More Stable Protein Formulations
Nanoparticulate impurities in pharmaceutical-grade sucrose
Nanoparticle impurities (NPIs) with a size of 100–300 nm have been discovered in pharmaceutical-grade sucrose (Weinbuch et al., 2015). These can lead to false analytical results, as they mimic protein aggregates and can thus cause the potential exclusion of “lead molecules” during early development stages. Studies have also shown that NPIs reduce the stability of final protein formulations by inducing protein aggregation, fragmentation and particle formation (Weinbuch et al., 2017).

This research was conducted to:
- Investigate the impact of NPIs isolated from beet- or cane-derived sucrose on drug product stability.
- Develop a purification process to reduce the amount of NPIs in sucrose.
Impact of nanoparticulate impurities on protein stability
Isolation of NPIs
NPIs were isolated from beet and cane derived sucrose. 50% sucrose solutions (w/v) were prepared in Milli-Q® water and diafiltration was performed against Milli-Q® water (6-fold volume exchange).
NPI spiking and forced degradation study
Isolated NPIs were spiked into IgG1 antibody mAbC formulation, resulting in a final particle concentration of ~1010 particles/mL. The formulations are summarized in Table 1.
Storage and stress conditions as well as time points for sample analysis are described in Table 2.
The stability of mAbC was assessed by using micro-flow imaging (MFI) using an MFI5200 system (ProteinSimple, Santa Clara, CA, USA) equipped with a 100-μm flow cell.
NPIs have a negative impact on protein stability
mAbC Formulations in the presence and absence of NPIs
Spiking with NPIs in a concentration of ~ 1010 particles/mL induced particle formation in mAbC formulation under stress conditions (Figure 1). This indicates that NPIs, independent of the sucrose source, can have a negative impact on protein stability in final drug product.

Figure 1.Results of forced degradation studies using mAbC without or with NPIs spiked that were previously isolated from beet- or cane-derived sucrose. Particle concentration was determined using MFI.
Purification of sucrose
Particle size analysis and quantification
10% sucrose solutions (w/v) were prepared in Milli-Q® water.
The solutions were measured before and after sterile filtration through a 0.22 μm PVDF membrane.
For Dynamic Light Scattering (DLS), samples were analyzed with the Zetasizer Nano series (Malvern, Herrenberg, Germany) at 25 °C using automatic attenuation selection and detection via 173 ° backscatter. Peak size was based on the viscosity of water as dispersant; particle area (%) was based on the intensity. The data processing analysis model was set to general purpose.
Nanoparticle Tracking Analysis (NTA) was performed with a NanoSight LM20 (NanoSight, Amesbury, UK) using a pre-run volume of 0.5 mL and triplicate measurements with 0.1 ml sample volume.
Quantification of β-Glucan contamination
50% sucrose solutions (w/v) were prepared in Milli-Q® water.
The (1→3)-β-D-glucan levels were measured by using the Glucatell® Assay (Cape Cod, East Falmout, MA, USA), according to the instructions of the manufacturer.
Purification process of sucrose
In order to mitigate risks during formulation development, nanoparticulate impurities were removed from sucrose in an improved purification process (Figure 2). This results in a sucrose grade that is low in NPI content, Sucrose Emprove® Expert. Additionally, the purification leads to a reduction of bioburden and endotoxin contamination.

Figure 2.Schematic overview of the improved purification process of sucrose in order to reduce nanoparticulate impurities as well as bioburden and endotoxin content.
Reduction of nanoparticulate impurities
Qualitative Analysis by DLS
- The first peak at about 1–5 nm is assigned to sucrose (Figure 3). The second peak, with a size distribution of about 100–300 nm, represents the NPIs.
- The decrease in the second peak (Figure 3) for Sucrose Emprove® Expert proves that the purification process successfully reduces NPI contamination in sucrose products.
- The remaining signal (Figure 3) around 100-300 nm can result from single larger particles, since the scattering intensity is proportional to diameter6 (I ~ d6).

Figure 3.DLS results for different batches of the non-purified sucrose raw material (left), and purified Sucrose Emprove® (right). Samples were measured in triplicates and error bars represent standard deviation.
Quantitative Analysis by NTA
The DLS results were confirmed by quantitative NTA measurements, which showed a significantly lower particle concentration in the four purified Sucrose Emprove® Expert batches compared to the non-purified raw material sucrose (Figure 4).
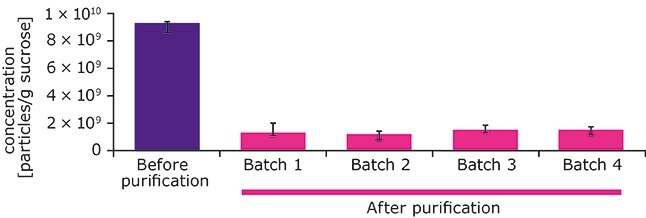
Figure 4.Total particle concentration of non-purified raw material sucrose compared to means of batches 1-4 of purified, low-NPI Sucrose Emprove® Expert. Error bars represent standard deviation from three replicate measurements.
Reduction of β-Glucan contamination
(1→3)-β-D-glucans can elicit inflammatory response and are potential contaminants in pharmaceutical product, originating from various raw material (Barton et al., 2016). The (1→3)-β-D-glucan levels were measured by using the Glucatell® assay that is based on a modification of Limulus Amebocyte Lysate (LAL) pathway, whereas factor C is eliminated. For this reason, this assay is specific for (1→3)-β-D-glucan (Figure 5).

Figure 5.Limulus Amebocyte Lysate (LAL) pathway. Factor C is depleted in Glucatell® assay to ensure specificity for (1→3)-β-D-glucan
In comparison to the non-purified raw material sucrose, the four purified Emprove® Expert batches contain a significantly lower amount of (1→3)-β-D-glucan, close to the detection limit of the Glucatell® assay. Thus, in addition to NPIs, (1→3)-β-D-glucan contaminants are also reduced during the purification process of sucrose.
Quantification by Glucatell® assay

Figure 6.Determination of (1→3)-β-D-glucan amount by using the Glucatell® assay. Duplicate measurements were carried out for each sample. The error bar represents the standard deviation.
Pharmaceutical-grade sucrose low in nanoparticulate impurities
Nanoparticle impurities (NPIs) have been discovered in pharmaceutical-grade sucrose in an amount up to 1010 particles per gram sucrose, resulting in false analytical results and in protein instability.
A purification process was successfully developed to reduce NPIs in sucrose, which was accompanied with a decrease of endotoxin, bioburden and β-D-glucan. This has enabled the development of a sucrose grade with unique quality characteristics, Sucrose Emprove® Expert Ph Eur, ChP, JP, NF.
Joint development
Sucrose Emprove® Expert was developed in cooperation with Coriolis Pharma, Munich, Germany.
References
To continue reading please sign in or create an account.
Don't Have An Account?