161195
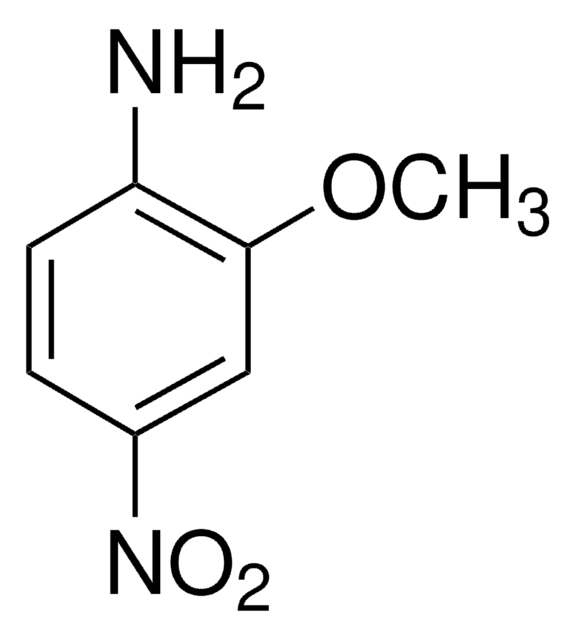
2-Methoxy-5-nitroaniline
98%
Synonym(s):
5-Nitro-o-anisidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H3(NO2)NH2
CAS Number:
Molecular Weight:
168.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
mp
117-119 °C (lit.)
SMILES string
COc1ccc(cc1N)[N+]([O-])=O
InChI
1S/C7H8N2O3/c1-12-7-3-2-5(9(10)11)4-6(7)8/h2-4H,8H2,1H3
InChI key
NIPDVSLAMPAWTP-UHFFFAOYSA-N
General description
2-Methoxy-5-nitroaniline is an aromatic metabolite of 2,4-dinitroanisole.
Application
2-Methoxy-5-nitroaniline was used in the synthesis of 5-(9-acridinylamino)-p-anisidines via reaction with 9-anilinoacridines. It was also used in the synthesis of disazo disperse dyes containing nitro and methoxy groups, used for the dyeing of polyester fibre.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
246.2 °F - closed cup
flash_point_c
119 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jidong Liang et al.
Journal of hazardous materials, 262, 281-287 (2013-09-18)
2,4-Dinitroanisole (DNAN) is an insensitive munitions compound considered to replace conventional explosives such as 2,4,6-trinitrotoluene (TNT). DNAN undergoes facile microbial reduction to 2-methoxy-5-nitroaniline (MENA) and 2,4-diaminoanisole (DAAN). This study investigated the inhibitory effect of DNAN, MENA, and DAAN toward various
5-Nitro-ortho-anisidine.
IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, 27, 133-139 (1982-04-01)
C Sawyer et al.
Biometrics, 40(1), 27-40 (1984-03-01)
An index of carcinogenic potency for chemicals tested in chronic animal experiments is described. By analogy with the well-known 'lethal dose 50' (LD50) of quantal bioassay, a 'tumorigenic dose 50' (TD50) may be defined (in the absence both of tumors
A Dewanji et al.
Biometrics, 49(2), 367-377 (1993-06-01)
In this paper, a new method of estimating tumorigenic potency is proposed that takes into account information on survival and, when available, the underlying cause of death. Specifically, Weibull distributions are used to describe the time to tumor occurrence (X)
Valeriy A Bacherikov et al.
Bioorganic & medicinal chemistry, 13(23), 6513-6520 (2005-09-06)
A series of 5-(9-acridinylamino)anisidines were synthesized by condensing methoxy-substituted 1,3-phenylenediamines (10 and 11) with 9-chloroacridine derivatives to form 5-(9-acridinylamino)-m-anisidines (AMAs, 14a-e) and 5-(9-acridinylamino)-o-anisidines (AOAs, 15a-e). 5-(9-Acridinylamino)-p-anisidines (APAs, 17a-e) were synthesized by reacting 2-methoxy-5-nitroaniline (12) with 9-anilinoacridines, followed by reduction. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service