All Photos(2)
About This Item
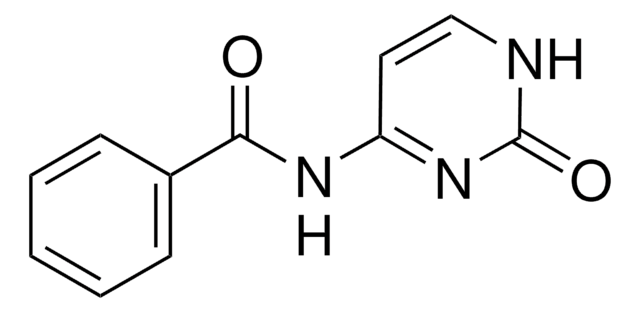
Empirical Formula (Hill Notation):
C6H7N3O2
CAS Number:
Molecular Weight:
153.14
Beilstein:
138451
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
>300 °C (lit.)
SMILES string
CC(=O)NC1=NC(=O)NC=C1
InChI
1S/C6H7N3O2/c1-4(10)8-5-2-3-7-6(11)9-5/h2-3H,1H3,(H2,7,8,9,10,11)
InChI key
IJCKBIINTQEGLY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Four stable conformers of N4-acetylcytosine, and the one of the stable form containing an intramoleuclar six-membered ring have been reported by theoretical calculations.The infrared and FT-Raman spectra of N4-acetylcytosine in the solid phase has been reported.
Application
N4-Acetylcytosine may be used in the preparation of cytidine. It may be used in the preparation of 1-(4-azido-4-deoxy-β-D-glucopyranosyl)cytosine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Dapeng Zhou et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 112, 139-145 (2013-05-15)
The infrared and FT-Raman spectra of N4-acetylcytosine in the solid phase have been recorded, and the molecular geometrical parameters were optimized using B3LYP and MP2 methods with 6-311++G(d,p) basis set. The theoretical calculations indicate the presence of four stable conformers
Synthesis and properties of N-(2, 3, 5-tri-O-acetyl-D-ribofuranosyl) maleimide.
Schwartz AL and Lerner LM.
The Journal of Organic Chemistry, 40(1), 24-28 (1975)
Nucleosides. 63. Synthetic studies on nucleoside antibiotics. 3. Total synthesis of 1-(4-amino-4-deoxy-beta-d-glucopyranosyluronic acid)cytosine, the nucleoside moiety of gougerotin.
K A Watanabe et al.
The Journal of organic chemistry, 35(1), 231-236 (1970-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service