All Photos(2)
About This Item
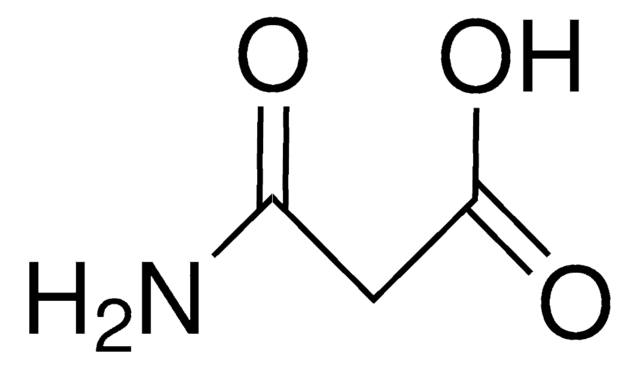
Linear Formula:
CH2(CONH2)2
CAS Number:
Molecular Weight:
102.09
Beilstein:
1751401
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
172-175 °C (lit.)
fluorescence
λex 367 nm; λem 445 nm (α-keto acid adduct)
SMILES string
NC(=O)CC(N)=O
InChI
1S/C3H6N2O2/c4-2(6)1-3(5)7/h1H2,(H2,4,6)(H2,5,7)
InChI key
WRIRWRKPLXCTFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The malonamide derivatives are obtained by the one-pot, five-component condensation reaction of isocyanide, Meldrum′s acid, arylidene malononitrile, and two amine molecules in CH2Cl2.
Application
The malonamide-based ionic liquid extractant was used in the extraction of europium(iii) and other trivalent rare-earth ions from nitric acid medium.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P J Blackall et al.
Veterinary microbiology, 11(3), 301-306 (1986-03-01)
The effect of using two different techniques for the detection of substrate alkalinization by Bordetella avium and reference strains of Alcaligenes faecalis and B. bronchiseptica was evaluated. The techniques used were those described by Otto and Pickett and Hinz et
Alok Rout et al.
Journal of hazardous materials, 221-222, 62-67 (2012-05-01)
The extraction behavior of U(VI), Pu(IV) and Am(III) from nitric acid medium by a solution of N,N-dimethyl-N,N-dioctyl-2-(2-hexyloxyethyl)malonamide (DMDOHEMA) in the room temperature ionic liquid, 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide (C(4)mimNTf(2)), was studied. The distribution ratio of these actinides in DMDOHEMA/C(4)mimNTf(2) was measured as
Bevin W Parks et al.
Inorganic chemistry, 45(4), 1498-1507 (2006-02-14)
This report describes an investigation into the coordination chemistry of trivalent lanthanides in solution and the solid state with acyclic and preorganized bicyclic malonamide ligands. Two experimental investigations were performed: solution binding affinities were determined through single-phase spectrophotometric titrations and
Abbas Rahmati et al.
Molecular diversity, 17(3), 619-625 (2013-05-25)
A one-pot, five-component condensation reaction of isocyanide, Meldrum's acid, arylidene malononitrile, and two amine molecules in CH2Cl2 at ambient temperature to give malonamide derivatives is described.
Jacqueline C Hargis et al.
Journal of the American Chemical Society, 130(51), 17471-17478 (2008-12-04)
A systematic study of various derivatives of malonaldehyde has been carried out to explore very short hydrogen bonds (r(OO) < 2.450 A). Various electron-withdrawing groups, including CN, NO(2), and BH(2), have been attached to the central carbon atom, C(2). To
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service