推薦產品
化驗
99%
bp
325-330 °C (lit.)
mp
118-120 °C (lit.)
SMILES 字串
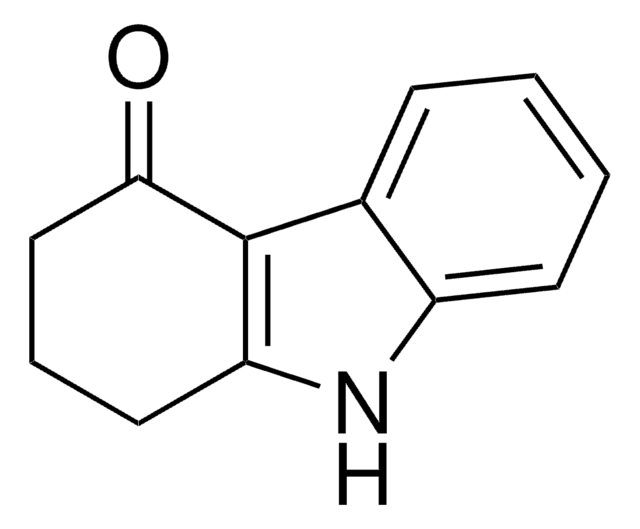
C1CCc2c(C1)[nH]c3ccccc23
InChI
1S/C12H13N/c1-3-7-11-9(5-1)10-6-2-4-8-12(10)13-11/h1,3,5,7,13H,2,4,6,8H2
InChI 密鑰
XKLNOVWDVMWTOB-UHFFFAOYSA-N
應用
1,2,3,4-四氢咔唑可作为起始材料:
- 通过光氧化反应制备螺[环戊烷-1,2′-吲哚啉-3′-酮]。
- 通过N-酰基化反应制备9-酰基-1,2,3,4-四氢咔唑。
- 通过钯催化的不对称加氢反应制备咔唑。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
J Cao et al.
Chemosphere, 40(12), 1411-1416 (2000-05-02)
The solubilization of four pairs of substituted indole compounds (SICs) by beta-cyclodextrin (beta-CD) in water was investigated. The results show that 1,2,3,4-tetrahydrocarbazole and N-methyl-1,2,3,4-tetrahydrocarbazole form 1:1 inclusion complexes with beta-CD, while the other six SICs form 1:2 inclusion complexes, respectively.
Chao Zheng et al.
Accounts of chemical research, 53(4), 974-987 (2020-04-11)
ConspectusThe Pictet-Spengler reaction is a fundamental named reaction in organic chemistry, and it is the most straightforward method for the synthesis of tetrahydro-β-carbolines, a core structure embedded in numerous alkaloids. Spiroindolenines are often proposed as possible intermediates in Pictet-Spengler reactions.
Subhasish Neogi et al.
Journal of combinatorial chemistry, 12(5), 617-629 (2010-07-01)
The one-pot synthesis of a new substituted 1,2,3,4-tetrahydrocarbazoles has been described via Petasis reactions. These tetrahydrocarbazoles exhibits various medicinal importance and might be suitable for elaboration into larger peptides at carboxy termini. The scope and limitations of this method have
Romano Di Fabio et al.
Bioorganic & medicinal chemistry letters, 16(6), 1749-1752 (2005-12-21)
The SAR of a new series of tetrahydrocarbazole derivatives is described: the appropriate decoration of this template led to the identification of a new class of NPY-1 antagonists showing good in vitro potency and a promising in vivo pharmacokinetic profile
Xu-Fan Wang et al.
Organic letters, 12(5), 1140-1143 (2010-02-12)
A hydrogen bonding-mediated double Michael addition-aromatization cascade of 2-propenylindoles and nitroolefins has been disclosed. The methodology allows an efficient synthesis of diverse and structurally complex tetrahydrocarbazoles in good to excellent enantioselectivities and diastereoselectivities.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務