Ultrafiltration/Diafiltration (UF/DF) in Plasmid DNA Manufacturing
Plasmid DNA (pDNA) production for mRNA, Plasmid-based DNA Vaccines, and Viral Vector Applications, involves a series of steps to grow, harvest, lyse, purify, and concentrate the DNA for further applications. Chromatography is used as part of the plasmid purification process, but the pDNA is often diluted during this step. At this stage, the plasmid is also often in an inappropriate buffer for further applications. This is where ultrafiltration and diafiltration (UF/DF) come in.
How are Ultrafiltration and Diafiltration Used in pDNA Production?
During UF/DF, the plasmid is concentrated and diafiltered in the appropriate buffer. This is typically accomplished by using tangential flow filtration (TFF) as this technique is easily scalable, highly selective, and cost-effective.
Ultrafiltration
During ultrafiltration, the molecules in solution are separated based on a size cutoff. Choosing a membrane pore size that can retain the plasmid but allow water, buffers, salts, and other chemicals to pass through allows the plasmid to be concentrated.
Diafiltration
In diafiltration, the chromatography elution buffer is exchanged for the final buffer in the same volume. This step occurs after the plasmid is concentrated using ultrafiltration. The final buffer is added to the feed as the chromatography buffer is diluted out.
Tangential Flow Filtration
Both ultrafiltration and diafiltration are performed under tangential flow filtration. In tangential flow filtration, the feed flows parallel to the membrane instead of through the membrane. By allowing the particles to flow perpendicular to the membrane surface, the material is less likely to clog up the filter.
Because the starting concentration of plasmids is generally much lower than that of a typical antibody or recombinant protein feed stream, use of TFF prior to chromatography also functions as a concentration step to further improve downstream purification.
Optimizing Ultrafiltration / Diafiltration Parameters
This membrane-based separation and concentration step needs to be optimized to achieve high performance without compromising the plasmid integrity. The performance of a TFF step depends on the feed conditions, molecular weight cut-off (MWCO), feed and filtrate/permeate flux, and system pressure. The desired plasmid purity, formulation, and concentration specification without product damage can be achieved through optimization of these hydraulic parameters. Additionally, air introduction needs to be avoided at all times, since air-liquid interphases can impact the integrity of the plasmid.
Improving Plasmid Retention During TFF
TFF relies on the size difference between pDNA and contaminants present in the lysate such as linear DNA, RNA and endotoxins. Therefore, the TFF membrane must have an appropriate MWCO to retain the pDNA and allow sieving of contaminants and the initial buffer. Due to their structure, plasmids can sometimes pass through pores that are smaller than their apparent molecular weight. This sieving can be more predominant with flux increase. The sieving coefficient also increases at higher ionic strength due to reduction in the effective plasmid size observed in these conditions1.
Higher salt concentration has been shown to reduce the plasmid radius1. In these conditions, the plasmid structure seems to be more tightly twisted, exhibiting a condensed effective size.
Loss of the pDNA in the permeate can potentially be addressed by polarizing the membrane (using full recirculation mode with permeate diverted into the feed tank) prior to starting the TFF run with the permeate line directed to exhaust. This will create a stable polarization layer that will improve the retention.
Managing Increased Viscosity
In addition to these retention and purification capabilities, TFF should be able to manage the increased viscosity throughout the process step and have a high capacity to enable an acceptable footprint at scale.
As viscosity also increases, particularly at concentrations approaching and exceeding 10 mg/mL, tight screens are not recommended. Coarse C-screen or suspended V-screen TFF device configurations should be applied for medium (5–10 X) to higher concentration (30–50 X) activities; TFF process optimization is required, however.
Optimizing the Transmembrane Pressure and Flux
In terms of parameters, a lower TMP is favored. Use of a two-pump, permeate-controlled system may be preferred for 100 kD and larger MWCO2. Depending on the specific configuration of the membrane used, the step is typically operated at TMP ≤10 psi for a permeate flux of ~20-50 L/ m²/h (LMH). The plasmid is usually completely retained at low filtrate flux and sieving can be observed at higher fluxes3.
The feed flux chosen for the concentration and diafiltration typically ranges between 4 and 6 L/min/m2 (LMM) to reduce mechanical stress that can ultimately lead to DNA degradation. High loading in the range of 70 to 140 L/m2 can be achieved if these pressure and flux parameters are well optimized with the correct membrane.
These typical operating parameters are summarized in Table 1.
Case Study: Buffer Exchange Using the Pellicon® 2 cassette with 300 kD Ultracel® membrane for Ultrafiltration/Diafiltration (UF/DF) of plasmid DNA
Objective: Buffer exchange using tangential flow filtration (TFF).
Pellicon® 2 cassettes with Biomax® membranes or Ultracel® membranes and C-screen or V-screen can be used for concentration and diafiltration of pDNA with high loading and yield. The V-screen configuration is specifically recommended for high concentration or high viscosity feed streams. For single-use applications, Pellicon® Capsules with Ultracel® membrane and C-screen are an optimal alternative. The selected MWCO depends on the pDNA structure and can range from 30 kD to 300 kD. The standard rule of thumb is to use a membrane cutoff that is 3–5 X tighter in pore diameter than the diameter of the product of interest. For common plasmid sizes of 5–20 kbp, 100 kD is often selected.
Materials
- Device - Pellicon® cassette, size 88 cm2
- Membrane - 300 kD Ultracel® (composite regenerated cellulose) membrane; retains ~10 kD DNA while allowing water, ionic and low molecular weight impurities to pass through
- Feed Screen - C-screen was selected for moderate viscosities
Buffer Exchange Process
- Membrane preparation
• Water flush: 20 L/m2
• Clean-in-place recirculation: 0.2 N NaOH, 20 L/m2 single pass
• Buffer flush: 20 L/m2 10 mM Tris, 1 mM EDTA, pH 8 (TE) - Concentration step
• Tank volume reduction with permeate to waste - Diafiltration step
• Constant volume diafiltration: add TE buffer at same rate as permeate removal - Recovery step
• Drain tank, then flush retentate line and tank
Buffer Exchange Results
The diafiltration buffer exchange was performed using a 300 kD Ultracel® membrane to retain the pDNA and pass water and low molecular weight impurities. A critical flux experiment at a 5 to 8 LMM crossflow was performed to show high 80-125 LMH fluxes were acceptable without fouling. Constant volume diafiltration at 4 LMM for 5 DV into a TE buffer showed declining TMP (no fouling). A retentate flush showed a 96% yield.
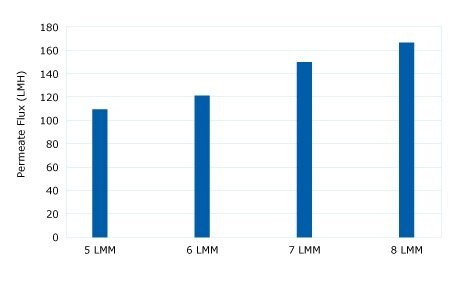
Figure 1.Critical Flux Evaluation
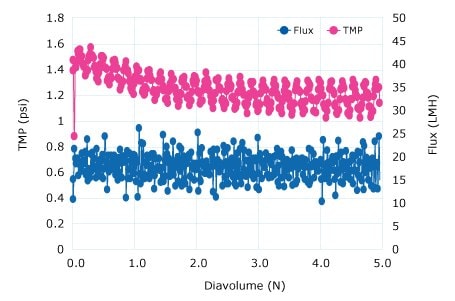
Figure 2.Diafiltration Run
Maintaining compliance with current Good Manufacturing Practices (cGMP’s) at any given time in your drug manufacturing process can be a complex and challenging task. To facilitate and accelerate your risk assessment continuum process, learn more about our Emprove® Program.
References
Para continuar lendo, faça login ou crie uma conta.
Ainda não tem uma conta?