T0256
Trypsin inhibitor from bovine pancreas
Type I-P, essentially salt-free, lyophilized powder
Synonym(s):
BPTI
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
bovine pancreas
type
Type I-P
form
essentially salt-free, lyophilized powder
mol wt
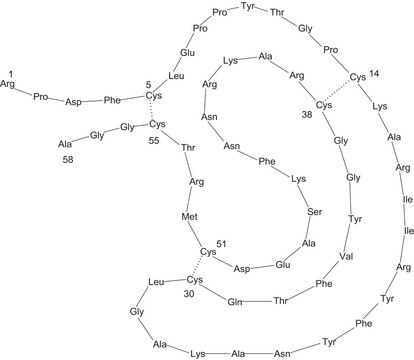
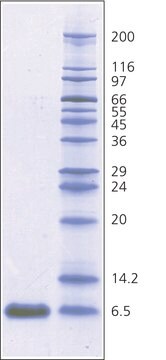
~6500 Da (single-chain 58-amino acid peptide)
purified by
crystallization
technique(s)
inhibition assay: suitable
solubility
water: 10 g/L
storage temp.
−20°C
Biochem/physiol Actions
A 58-residue protein whose binding to various serine proteases has been extensively studied. It is highly homologous to a family of mamba snake dendrotoxin proteins that inhibit various K+ channels. It also binds to an intracellular site associated with the large conductance Ca2+-activated K+ channel..
Unit Definition
One trypsin unit will produce a ΔA253 of 0.001 per min with BAEE as substrate at pH 7.6 at 25 °C; reaction volume 3.2 ml, 1 cm light path.
Preparation Note
Prepared by method of Kunitz and Northrup, J. Gen. Physiol., 19, 991 (1936).
Analysis Note
One mg of trypsin inhibitor will inhibit greater than 1.5 mg of trypsin with activity of approximately 10,000 BAEE units per mg protein.
Protein determined by biuret.
Other Notes
View more information on Trypsin Inhibitor.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alexandre P Alloy et al.
The Journal of biological chemistry, 290(35), 21523-21535 (2015-07-16)
Human mesotrypsin is highly homologous to other mammalian trypsins, and yet it is functionally unique in possessing resistance to inhibition by canonical serine protease inhibitors and in cleaving these inhibitors as preferred substrates. Arg-193 and Ser-39 have been identified as
Fanlu Meng et al.
Journal of plant research, 131(5), 827-837 (2018-05-08)
PeaT1 is a proteinaceous elicitor from fungal pathogen Alternaria tenuissima. Our previous research revealed that this elicitor could induce defense response and enhance disease resistance in various plants including Nicotiana plants. However, immune activation mechanisms whereby PeaT1 elicits defense response
J N Whitaker et al.
The Biochemical journal, 201(3), 543-553 (1982-03-01)
Previous studies have demonstrated that the kidney is the major site for clearance and catabolism of a peptide (residues 43-88) of encephalitogenic or basic protein (BP) derived from central-nervous-system myelin. In the present investigation rat renal tissue was shown to
Martina Holubová et al.
The Journal of pharmacology and experimental therapeutics, 366(3), 422-432 (2018-06-20)
Ghrelin, the only known orexigenic gut hormone produced primarily in the stomach, has lately gained attention as a potential treatment of anorexia and cachexia. However, its biologic stability is highly limited; therefore, a number of both peptide and nonpeptide ghrelin
C L Dumke et al.
Journal of applied physiology (Bethesda, Md. : 1985), 92(2), 657-664 (2002-01-18)
Serum proteins [molecular weight (MW) > 10,000] are essential for increased insulin-stimulated glucose transport after in vitro muscle contractions. We investigated the role of the kallikrein-kininogen system, including bradykinin, which is derived from kallikrein (MW > 10,000)-catalyzed degradation of serum
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service