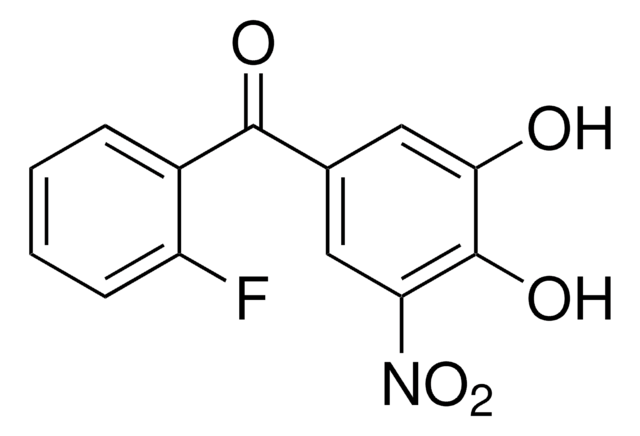
SML2053
Lalistat 2
≥98% (HPLC)
Synonym(s):
1-Piperidinecarboxylic acid 4-(1-piperidinyl)-1,2,5-thiadiazol-3-yl ester, Lalistat-2
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H20N4O2S
CAS Number:
Molecular Weight:
296.39
MDL number:
UNSPSC Code:
41106300
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
2-8°C
SMILES string
O=C(N1CCCCC1)OC2=NSN=C2N3CCCCC3
Application
Lalistat 2 has been used as a lysosomal acid lipase (LAL) inhibitor to study its effects on hypoxia-inducible factor (HIF) in mice. It has also been used as a LAL inhibitor to study LAL activity on dried blood spot cards.
Biochem/physiol Actions
Lalistat-2 is a potent and specific competitive inhibitor of the lysosomal acid lipase (LAL/Lipa). Lalistat-2 affects lipid droplets morphology and localization.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maidina Tuohetahuntila et al.
The Journal of biological chemistry, 292(30), 12436-12448 (2017-06-16)
Activation of hepatic stellate cells (HSCs) is a critical step in the development of liver fibrosis. During activation, HSCs lose their lipid droplets (LDs) containing triacylglycerols (TAGs), cholesteryl esters, and retinyl esters (REs). We previously provided evidence for the presence
Stefanie Schlager et al.
Oncotarget, 8(25), 40037-40051 (2017-04-14)
Degradation of lysosomal lipids requires lysosomal acid lipase (LAL), the only intracellular lipase known to be active at acidic pH. We found LAL to be expressed in murine immune cells with highest mRNA expression in macrophages and neutrophils. Furthermore, we
Chiara Pavanello et al.
Pharmacological research, 147, 104362-104362 (2019-07-23)
Lysosomal acid lipase (LAL) is responsible for the hydrolysis of cholesteryl esters (CE) and triglycerides (TG) within the lysosomes; generated cholesterol and free fatty acids (FFA) are released in the cytosol where they can regulate their own synthesis and metabolism.
Anton I Rosenbaum et al.
Journal of medicinal chemistry, 53(14), 5281-5289 (2010-06-19)
Niemann-Pick type C (NPC) disease is a lysosomal storage disorder characterized at the cellular level by abnormal accumulation of cholesterol and other lipids in lysosomal storage organelles. Lysosomal acid lipase (LAL) has been recently identified as a potential therapeutic target
Xinlei Li et al.
Frontiers in cell and developmental biology, 9, 640667-640667 (2021-04-06)
Extracellular vesicles (EVs) are membrane-limited nanoparticles that are liberated by cells and contain a complex molecular payload comprising proteins, microRNA, RNAs, and lipids. EVs may be taken up by other cells resulting in their phenotypic or functional reprogramming. In the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service