All Photos(1)
About This Item
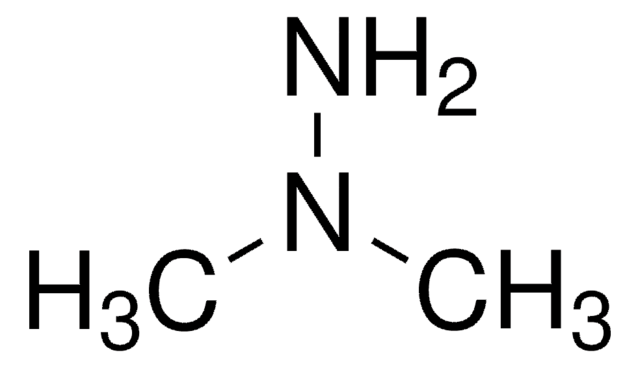
Linear Formula:
(CH3)2NCSN(CH3)2
CAS Number:
Molecular Weight:
132.23
Beilstein:
1744916
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
245 °C (lit.)
mp
75-77 °C (lit.)
storage temp.
2-8°C
SMILES string
CN(C)C(=S)N(C)C
InChI
1S/C5H12N2S/c1-6(2)5(8)7(3)4/h1-4H3
InChI key
MNOILHPDHOHILI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Tetramethylthiourea molecule shows crystallographic two-fold symmetry with weak hydrogen bond packing, which connects the molecules to form layers.
Application
Tetramethylthiourea forms BF3 adduct with tetramethylselenourea.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
I Neumcke et al.
Endocrinology, 140(2), 641-645 (1999-02-02)
The most important stimulus for the enhanced synthesis of erythropoietin (Epo) is a lowered O2 tension in the tissue. However, the mechanism by which an impaired O2 supply is transduced into appropriate Epo production is still not fully understood. Recently
Allan Godsk Larsen et al.
Journal of colloid and interface science, 279(1), 158-166 (2004-09-24)
The oxidation of tetramethylthiourea (TMTU) at gold electrodes in acetonitrile, leading to dissolution of the electrode, has been studied by electrochemical methods and by an electrochemical quartz crystal microbalance (EQCM). TMTU in acetonitrile readily adsorbs at gold electrodes and an
Yefeng Tang et al.
Organic letters, 7(4), 593-595 (2005-02-12)
A Pauson-Khand type of conversion of enynes to bicyclic cyclopentenones employing the commercially available Co2(CO)8 and tetramethylthiourea (TMTU) as catalysts is described. This method allows a variety of enynes with diverse functional groups to be cyclized into cyclopentenones of interest.
Urea-boron trihalide adducts. IV. Ionic-covalent equilibria in the tetramethylthiourea-BF3 and tetramethylselenourea-BF3 systems and the influence of B2F7-formation.
Hartman JS, et al.
Canadian Journal of Chemistry, 54(7), 1121-1129 (1976)
J Palassis
American Industrial Hygiene Association journal, 41(2), 91-97 (1980-02-01)
Tetramethyl and ethylene thiourea are collected from air using midget impingers containing 15 mL water. Ethylene thiourea may also be collected from air using PVC or cellulose ester membrane filters which are then extracted with water. Pentacyanoamineferrate reagent is added
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service