3D Mammosphere Culture Media
Mammospheres, or mammary epithelial stem cell aggregates, derived from primary breast tumors or cell lines are thought to develop from rare cancer stem cell (CSC) subpopulations within the tumor. The progression and recurrence of breast cancer, the leading cause of cancer death in women, has been linked to small populations of cancer stem cells (CSCs).1 The human mammary gland can undergo multiple cycles of changes in prooliferation and function controlled by stem cells. Signaling pathways involved in mammary stem cell regulation are often deregulated in breast cancer.1 These cancer stem cell populations express stem cell related markers Nestin and Sox2 while lacking GFAP (2). The 3D culture of breast-derived cancer cells is considered to be a more biologically relevant cell culture model maintaining the original malignant tumor phenotype.
Breast cancer cells cultured in fetal bovine serum (FBS) undergo unwanted phenotype changes and cell differentiation, while culturing 3D mammospheres in serum-free conditions maintains the original properties of the primary tumor.4 However, conventional serum-free media for culturing mammospheres struggle to efficiently maintain the self-renewing subpopulations of cancer stem cells (CSCs). Consequently, the stem cell population is gradually lost overtime along with the cell proliferation rates. The PromoCell 3D Tumorsphere Media XF (C-28070, C-28075) is designed to serially passage breast cancer derived cells as undifferentiated 3D mammospheres with high cell proliferation rates. It’s defined and serum-free formualtion allows consistent and standardized expansion of mammospheres while maintaining the cancer stem cell characteristics such as self-renewal and chemoresistance.
See Product References
Mammosphere Culture Protocol
Tumorsphere Formation
- Harvest the adherent cells. Deteach the breast cancer cell line using standard detachment protocols. The cells should be 80-90% confluent in log phase of growth. Centrifuge the cell suspension for 5 minutes at 300 x g and aspirate the supernatant. Resuspend the cells in 3-5 ml of fresh 3D Tumorsphere Media XF (C-28070, C-28075).
- Count the cells. Count the cells using your routine method and adjust the volume of media to obtain a concentration of 1 X 106 cell/ml..
- Set up the mammosphere culture. Seed the cells in appropriate suspension culture vessel (Corning ULA or Spheroid Plates) at 1 x 104 cell/ml in 4 mL of fresh 3D Tumorsphere Media XF (C-28070, C-28075) in a 6-well culture plate.
- Allow the mammospheres to grow. Incubate for 4-10 days depending on the cell type used. Add half the volume of media every 3-4 days. Do not change the entire volume of media.
Passaging Mammospheres
Passage the spheroid before they form a dark necrotic center (typically 4-10 days). Collect
- Collect the Mammospheres. Transfer the mammospheres into a 15 ml conical tube.
- Gravity sedimentation of mammospheres. Allow the spheres to settle by gravity sedimaentation for 10 minutes at room temperature. Aspirate the supernantant but leave approximately 200 µL in the conical tube. Do not aspirate mammospheres.
- Wash the mammospheres. Repeat step two with an equal volume of PBS (D8537). Gently asirate the PBS and leave 200 µL in the conical tube.
- Enzymatic digest the mammospheres. Add 1 mL of Trypsin-EDTA (T3924) to the mammospheres and incubate for 2-4 minutes at room temperatrue. Pipet up and down every 30 seconds to avoid sedimentation. Some cells require a longer incubation period.
- Break apart the mammospheres. Pipet up and down 10-20 times using a p-1000 to achieve a single cell suspension. Add twice the volume of Trypsin Neutralization Solution (T6414).
- Determine cell viability. Make up 5 mL with fresh 3D Tumorsphere Medium XF (C-28070, C-28075) and dtermine the cell number and viability. Centrifuge cells for 5 minutes at 300 x g. Discard supernatant and resuspend at 1 X 106 cells/mL in fresh media.
- Plate cells. Reseed the cells at 1X104 cells/mL in a new culture vessel (ie. 4X104 cells in 4 mL of media using a 6-well plate)
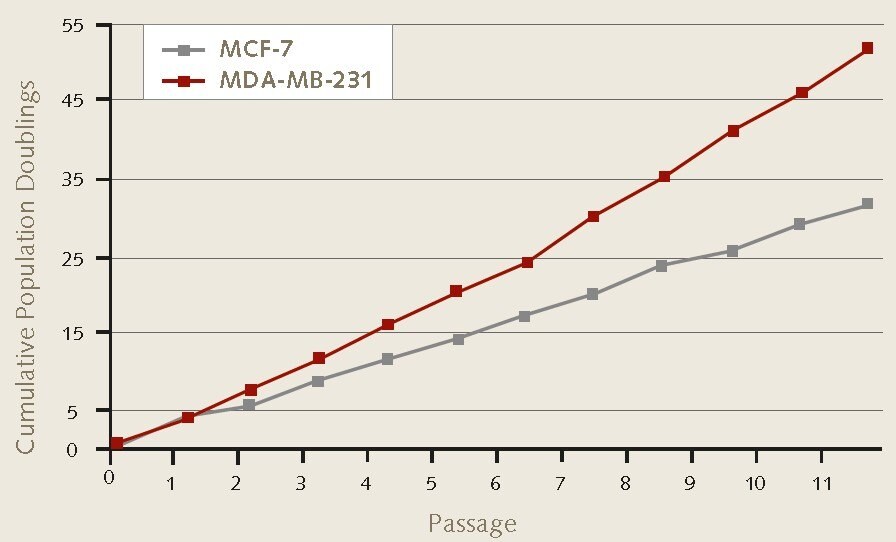
Figure 1. Cell proliferation rates of MCF-7 and MDA-MB-231 breast cancer cells during 3D mammosphere culture. 4X104 MCF-7 (86012803) or MDA-MB-231 (92020424) cells/well were plated in triplicate using 6-well ULA suspension plates. Serial passage by enzymatic dissociation was performed according to protocol. Mammosphere formation and cell proliferation were maintained during 3D culture over 11 passages.
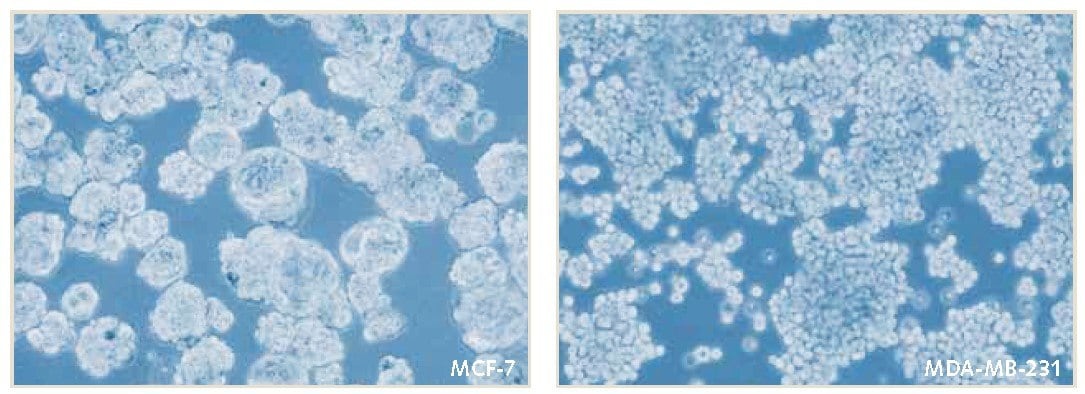
Figure 2. Mammosphere culture of MCF-7 and MDA-MB-231 breast cancer cells in PromoCell 3D Tumorsphere Media XF. Mammospheres were serially passaged by enzymatic dissociation was performed according to protocol. Mammosphere formation and cell proliferation were maintained during 3D culture over 11 passages.

Figure 3. Population doublings and tumor formation efficiencies of MCF-7 breast cancer cells during 3D mammosphere culture.A) 4X104 MCF-7 cells/well were plated in triplicate using 6-well ULA suspension plates. Serial passage by enzymatic dissociation was performed every 9 days. Consistent mammosphere formation and cell proliferation were maintained during 3D culture over 11 passages. B) Serial passage of MCF-7 cells over 9 passages results in significant increase in tumor formation efficiency from 2% to 28%.

Figure 4. Cell morghology of MDA-MB-231 breast cancer cells during 3D mammosphere culture.Mammospheres form non-adherent suspension aggregates in PromoCells’s Tumorsphere Media XF compared to unwanted adherent growth pattern in competitors tumorpshere media.
References
To continue reading please sign in or create an account.
Don't Have An Account?