Advanced Protocol: How to Customize your TrueGel3D™ Hydrogel
1-Addition of peptide
The identity and concentration of peptides or proteins can be varied when constructing a TrueGel 3D™ hydrogel. This peptide concentration optimization may be important for specific applications such as migration/motility or cell polarity assays. As an example, Lutolf et al. demonstrated the minimum concentration of RGD ligands necessary for fibroblast outgrowth1.
In the final hydrogel the concentration of reactive groups (SH-reactive groups) must be equivalent to the concentration of the SH-groups. If SH-functionalized peptides or proteins are included in the gel, the concentration of reactive groups of the polymer must be correlated with the sum of the SH-groups coming from the crosslinker plus the SH-groups coming from peptides/proteins.
If different concentrations of peptide are to be tested, you will need to add in your mix a corresponding amount of thioglycerol to ensure that the final strength of the gels you are comparing are the same (gel stiffness is correlated to the number of thiol groups in the hydrogel). A capacity for up to 5 mmol/L of peptides in the final hydrogel provides flexibility when varying the number and concentration of peptides.
TrueGel3D™ RGD integrin adhesion peptide concentration optimization
For the TrueGel3D™ RGD integrin adhesion peptide, it has been shown that a peptide concentration of 0.5 mmol/L peptide in the final hydrogel is sufficient to provide attachment sites for cells.
Table 1 indicates how to set up reaction mixes to achieve different concentrations of TrueGel3D™ RGD integrin adhesion peptide. Please refer to instructions that follow this table for use of other peptides.
* TrueGel3D™ RGD integrin adhesion peptide and TrueGel3D™ thioglycerol stock solution (20 mmol/L) are diluted to 3mmol/L to increase pipetting volumes.
Use of other types of peptides (custom or commercially available peptides)
You may use peptides other than TrueGel3D™ RGD integrin adhesion peptide. Eligible peptides must include a cysteine residue at the terminal end of the peptide (N or C terminal) to ensure the presence of a thiol group in the sequence (needed to covalently attach your peptide to the thiol-reactive PVA or dextran polymers). Having a positively-charged amino acid in the immediate proximity of the N-terminal cysteine should be avoided (a spacer may be incorporated to preclude this proximity). Peptide quality is an important parameter: use only purified (e.g. HPLC) peptide, aliquoted into a small-volume container with minimal headspace to avoid air oxidation.
2-Optimize stiffness/rigidity of your hydrogel
Your application may require a hydrogel of a specific stiffness, depending on the cells you are using and the nature of the assay. Gel firmness increases with an increasing concentration of thiol-reactive polymers and related crosslinker. This means that firmer gels can be obtained by increasing the concentrations of the thiol reactive polymer (PVA or dextran) and crosslinker (PEG non-cell-degradable crosslinker or CD cell-degradable crosslinker). The crosslinking strength is related to concentration of both polymers and crosslinker.
Table 2 allows you to prepare your reaction mix depending on desired gel firmness:
Gel stiffness may be adjusted by using a mixture of both fast and slow gelling hydrogels. For fast gelling hydrogels, the time between addition of crosslinker and beginning of solidification of the gel solution is shorter. Gelation can be virtually instant, and makes this optimization challenging, but possible.
Furthermore, the gelation time varies with the type of polymer (SLO-PVA or SLO-DEXTRAN) and the type of crosslinker (CD cell-degradable or PEG non-cell-degradable) used. Gelation times should be determined in preliminary tests without cells. Once gelation begins, the reaction should be allowed to proceed until no threads of gel can be pulled out from the surface of the gel when probed with a pipet tip. After this time, it should be possible to immerse the gel in culture medium without disruption of the gel structure.
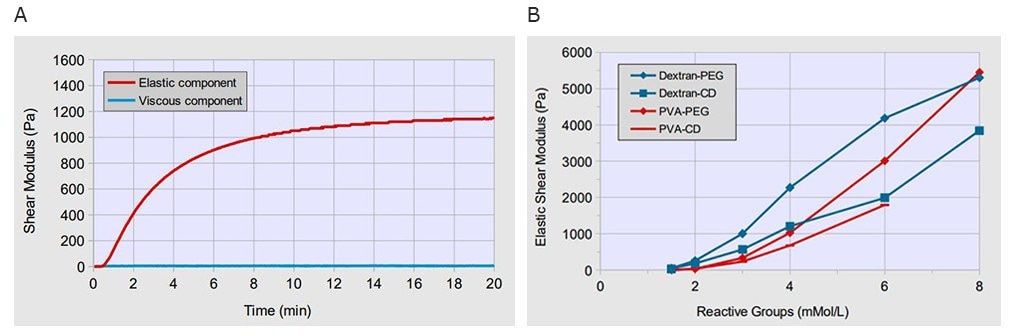
Figure 1.Hydrogel stiffness can be controlled using TrueGel3D synthetic hydrogels. A) Formation of hydrogel after mixing Dextran-Maleimide and PEG-Thiol crosslinkers at a final concentration of 3 mMol/L for both components. Within one minute after mixing a hydrogel forms which can be seen by the increasing elastic component of the shear modulus of the material. In contrast, the viscous component of the shear modulus remains low, below 10 Pascal (Pa), indicating the formation of a highly elastic hydrogel with only a small viscous share. B) Soft hydrogels with an elastic shear modulus of 20-40 Pa can be generated by mixing the polymer components at a final concentration of each reactive group of 1.5 mMol/L. Stiffer hydrogels with an elastic shear modulus of up to 4000-6000 Pa can be obtained at higher concentrations of reactive groups >6 mMol/L.
Reference
To continue reading please sign in or create an account.
Don't Have An Account?