Pyrogen Testing

Pyrogen testing determines the presence or absence of pyrogens in parenteral pharmaceutical products and is regulated by several standards from organizations such as the Food and Drug Administration (FDA), United States Pharmacopeia (USP), or European Pharmacopeia (EP). The sterility of a product does not imply that it is free of pyrogens. Therefore, drugs that are purported to be sterile must also be tested for pyrogens to prevent febrile reactions in patients.
Pyrogen contamination can occur during production or the administration of pharmaceuticals, biotherapeutics, and medical devices, but the presence of pyrogens can also be an inherent characteristic of the product, such as adjuvants in vaccines or synthetic lipopeptides.
Related Technical Articles

BIOSAFETY TESTING SERVICES
Cell line characterization services to confirm cell line species origin and history, as well as characterize and test cell line identity, genetic stability, and purity (including Pyrogen testing).
What is a Pyrogen?
A pyrogen is a substance that causes a rise in temperature (fever reaction) in a human or animal through the activation of the innate immune system. They constitute a heterogeneous group of contaminants comprising microbial and non-microbial substances. Pyrogens can be classified into two groups: endotoxins and non-endotoxin pyrogens (NEPs). Endotoxins are substances found in Gram-negative bacteria. Non-endotoxin pyrogens are other microbial substances, including those derived from Gram-positive bacteria or viruses and pyrogens originating from yeasts and fungi. Non-microbial pyrogenic substances can also originate from rubber particles, microscopic plastic particles, or metal compounds in elastomers.
Several test methods are available for the detection of pyrogens. They can be classified based on the type of contaminant they detect, and the need for animal materials to perform the test as described in the below table:
Rabbit Pyrogen Testing
The rabbit pyrogen test (RPT) involves measuring the rise in temperature of rabbits following the intravenous injection of the tested product. The RPT gives qualitative results and the sensitivity is quite low. The robustness of the test is also limited, due to development of pyrogen tolerance in rabbits after repeated injections, or stress from the rabbits when performing the assay.
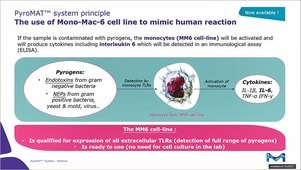
Monocyte Activation Test
The Monocyte Activation Test (MAT) is an alternative to animal-based methods for the detection of both endotoxin and non-endotoxin pyrogens. The monocyte activation test mimics the human immune reaction by incubating monocytes with the tested sample. If pyrogens are present, monocytes are activated and produce inflammatory molecules, cytokines, responsible for the febrile reaction. The cytokines are then detected using an immunological assay (ELISA) involving specific antibodies and an enzymatic color reaction.
Please Note: The European Pharmacopoeia (Ph. Eur.) Commission took the decision to engage on a path that should ultimately lead to the complete replacement of the rabbit pyrogen test (RPT) in the Ph. Eur., within approximately 5 years.
Read the article and discuss further with our Experts to start the move.
Bacterial Endotoxin Testing (LAL test)
The most common method for endotoxin testing is the Limulus amoebocyte lysate test (LAL test); an assay based on the lysate of amoebocytes from the horseshoe crab blood. The lysate from horseshoe crab blood cells naturally reacts with bacterial endotoxins in a coagulation reaction. This method has a high sensitivity for the quantification of endotoxins, but it does not detect non-endotoxin pyrogens.
Recombinant Factor C (rFC) Testing
The recombinant Factor C is a genetically engineered protein normally found in the Limulus amebocyte lysate cascade. In this test, Factor C reacts with endotoxin and couples with a marker to produce a quantifiable, fluorescent end product. The recombinant Factor C test uses the same principle as the LAL test, but without the need for animal-derived material.
Related Webinars
In this webinar, we discuss how monocyte activation tests performed with the PyroMAT® System detect endotoxin and non-endotoxin pyrogens.
Learn how our PyroMAT® System provides a robust solution for in vitro Pyrogen Test in pharma with a ready-to use kit.
In this lecture, you will learn how to test for pyrogen (including non-endotoxin pyrogens) in your pharmaceutical samples, what are the existing methods in order to have a controlled process.
To continue reading please sign in or create an account.
Don't Have An Account?