W108
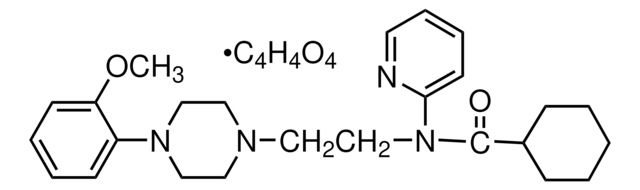
WAY-100635 maleate salt
powder
Synonym(s):
N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt
About This Item
Recommended Products
form
powder
Quality Level
color
white
solubility
H2O: 25 mg/mL
SMILES string
[H]\C(=C(/[H])C(O)=O)C(O)=O.COc1ccccc1N2CCN(CC2)CCN(C(=O)C3CCCCC3)c4ccccn4
InChI
1S/C25H34N4O2.C4H4O4/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21;5-3(6)1-2-4(7)8/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1-
InChI key
XIGAHNVCEFUYOV-BTJKTKAUSA-N
Gene Information
human ... HTR1A(3350)
Application
- to study whether the behavior of the cleaner wrasse is regulated by serotonin activity
- to block activity of 3,4 methylenedioxymethamphetamine (MDMA) used to induce oxytocin release in male Wistar rats to increase their social behavior
- to study the role of serotonin during metamorphosis of the ascidian Phallusia mammillata larvae
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service