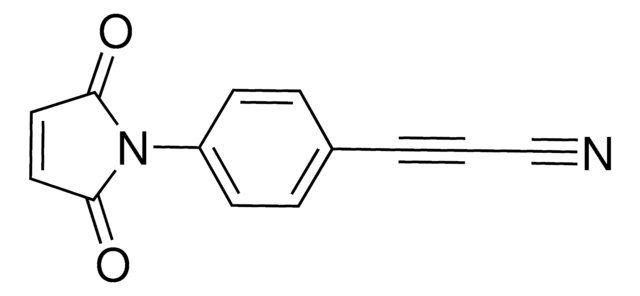
760668
Dibenzocyclooctyne-maleimide
for Copper-free Click Chemistry
Sinónimos:
DBCO-maleimide
About This Item
Productos recomendados
form
solid
reaction suitability
reaction type: click chemistry
reagent type: cross-linking reagent
functional group
maleimide
storage temp.
−20°C
SMILES string
O=C(N1CCC(NCCC(N2CC3=C(C=CC=C3)C#CC4=C2C=CC=C4)=O)=O)C=CC1=O
InChI
1S/C25H21N3O4/c29-22(14-16-27-23(30)11-12-24(27)31)26-15-13-25(32)28-17-20-7-2-1-5-18(20)9-10-19-6-3-4-8-21(19)28/h1-8,11-12H,13-17H2,(H,26,29)
InChI key
NHFQNAGPXIVKND-UHFFFAOYSA-N
Application
Applications Include:
- Protein-peptide conjugates
- Antibody-enzyme or antibody-drug conjugates
- Protein or peptide-oligonucleotide conjugates
- Surface modification
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico


![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)


![N-[(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/294/853/c5e47d84-5aee-4797-aa24-604f291171cc/640/c5e47d84-5aee-4797-aa24-604f291171cc.png)
![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethanol for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/171/632/0556139a-2db5-4678-a6ec-a26a693fd574/640/0556139a-2db5-4678-a6ec-a26a693fd574.png)