19671
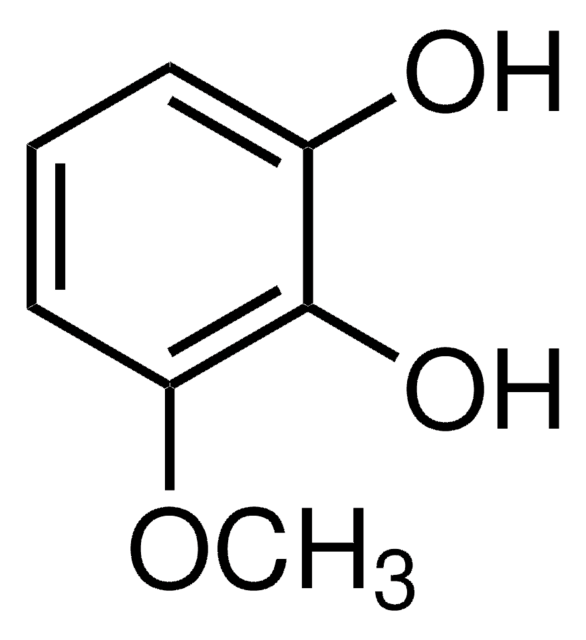
4-tert-Butylcatechol
≥99.0%
Sinónimos:
4-(1,1-Dimethylethyl)benzene-1,2-diol, 4-tert-Butylcatechin, 4-tert-Butylpyrocatechol, 4-TBC, p-tert-Butylcatechol
About This Item
Productos recomendados
Quality Level
assay
≥99.0%
form
powder
bp
285 °C (lit.)
mp
52-55 °C (lit.)
56-58 °C
solubility
methanol: soluble 1 g/10 mL, clear, colorless
SMILES string
CC(C)(C)c1ccc(O)c(O)c1
InChI
1S/C10H14O2/c1-10(2,3)7-4-5-8(11)9(12)6-7/h4-6,11-12H,1-3H3
InChI key
XESZUVZBAMCAEJ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Antioxidant source for radical reactions: Research identified 4-tert-Butylcatechol as a viable source of hydrogen atoms in radical reactions, specifically in deiodination processes, expanding its utility in synthetic organic chemistry (Povie et al., 2016).
signalword
Danger
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico