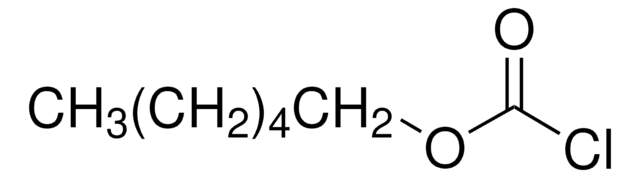185892
Ethyl chloroformate
97%
Sinónimos:
Chloroformic acid ethyl ester
About This Item
Productos recomendados
vapor density
3.74 (vs air)
Quality Level
vapor pressure
3.42 psi ( 20 °C)
assay
97%
form
liquid
autoignition temp.
842 °F
refractive index
n20/D 1.395 (lit.)
bp
93 °C (lit.)
mp
−81 °C (lit.)
solubility
alcohol: miscible
benzene: miscible
chloroform: miscible
diethyl ether: miscible
water: insoluble
density
1.135 g/mL at 25 °C (lit.)
SMILES string
CCOC(Cl)=O
InChI
1S/C3H5ClO2/c1-2-6-3(4)5/h2H2,1H3
InChI key
RIFGWPKJUGCATF-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- Ethyl chloroformate was used in the synthesis of nitrile oxides.
- It was used as derivatization reagent to investigate stereochemical conversion of chiral non-steroidal anti-inflammatory drugs during derivatization reaction.
- It was used to develop flexible template strategy for the generation of nanometer-scale templates via dip-pen nanolithography.
- It was used as derivatization reagent in an enantiomeric analysis of amino acids by two-dimensional gas chromatography.
- It was also used with ammonia and cyanuric chloride to convert carboxylic acids to nitriles.
Packaging
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Met. Corr. 1 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
60.8 °F
flash_point_c
16 °C
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico