Factors Affecting Analyte Binding on Membrane Filters: A Guide for Filter Validation in Pharmaceutical Analytical QC
Summary:
The following Millex® syringe filters demonstrated low analyte binding of the APIs in the drug formulations used in this study.
Acetaminophen and acetyl salicylic acid showed strong binding to Millex® filters containing nylon membrane.
Sample filtration in compendial methods
Sample filtration finds applications in compendial methods, such as the United States Pharmacopeia (USP) and the European Pharmacopeia (Ph. Eur.). One such application is Dissolution Test for solid dosage forms, wherein filtration removes undissolved drug particles and excipients from the withdrawn sample. If these particles remain in the sample solution, they have the potential to continue dissolving and result to inaccurate data. The USP <1092> The Dissolution Procedure: Development and Validation includes a section on Performing Filter Compatibility. It states:
“Selection of the proper filter material is important and should be accomplished, and experimentally justified, early in the development of the dissolution procedure.”1
In Ph. Eur. 2.9.3 Dissolution Test for Solid Dosage Forms, a note on the Procedure for Apparatus 1 and 2 states:
“Test specimens are filtered immediately upon sampling unless filtration is demonstrated to be unnecessary. Use an inert filter that does not cause adsorption of the active substance or contain extractable substances that would interfere with the analysis.”2
Monographs that involve HPLC analysis, such as the assay used to measure the concentration of the active pharmaceutical ingredient (API) and the quantification of organic impurities or related substances, often include a step of filtering the test solution prior to injection into the instrument. This filtration step ensures the removal of undissolved particles that can potentially clog or damage the HPLC column, leading to system issues and inaccurate results.3
Sample filtration plays a crucial role in analytical procedures that use it as part of the sample preparation step. The filtration device used in a method should not compromise the accuracy and reproducibility of data. Therefore, filter validation studies should be used to evaluate analyte loss due to membrane filter adsorption.
Analyte binding to membrane filters
Different membrane filters can bind analyte to varying degrees depending on membrane and analyte type, as well as analyte concentration.
The objective of this study was to provide guidance on filter selection during method development and validation with a special emphasis on analyte binding to syringe filters. The following membrane and analyte characteristics were evaluated as part of this study:
- Choice of membranes
- Effect of physico-chemical properties of analyte
- Effect of analyte concentration
- Membrane pore size
- Processing conditions and effect on analyte recovery
Experimental methods
Part 1. Evaluation of analyte adsorption through an API recovery study
Drug dissolution studies were performed using multiple commercially available formulations and methods outlined in respective USP monographs. Samples were filtered using different syringe filters and various filtrate fractions were collected. Filtrate was analyzed by HPLC for quantitation of active pharmaceutical ingredient (API). Centrifuged samples were used as controls for 100% analyte recovery to calculate analyte binding to syringe filters. Table 1 lists formulations used in this study. Table 2 lists the dissolution and HPLC methods used.
Similar studies were also conducted on a blend uniformity sample provided by one of our customers. The sample was dissolved in a solvent blend and filtered through various syringe filters. HPLC analysis of the filtrate was carried out and recovery was calculated using a standard prepared in the same way.
Part 2. Volumetric sample recovery
Volumetric sample recovery from various syringe filters was determined by filtration of a fixed volume of sample through the syringe filter and measuring the volume of liquid collected in a vial. This provided information about volume retained by a syringe filter and its impact on analyte binding.
Results and Discussion
The introduction of variability and inaccuracies in analytical results can occur due to analyte loss during sample filtration. Therefore, it is important to understand the factors that contribute to analyte loss in order to establish robust analytical methods and effectively mitigate this issue.
Physico-chemical Properties of Analytes and Membranes
Analyte binding is mostly dependent on physico-chemical properties of both the membrane and analyte, since binding results from various secondary interactions between analyte and membrane. Some common secondary interactions that lead to analyte binding are electrostatic interactions, hydrogen bonding, and hydrophobic interactions. Figure 1 shows binding of different analytes from a multi-component migraine formulation using three different Millex® syringe filters containing hydrophilic PTFE, Durapore® PVDF, or nylon membranes. Active Pharmaceutical Ingredients (APIs) present in this formulation had different physico-chemical properties. This formulation contains an acidic (acetyl salicylic acid), neutral (caffeine), and basic (acetaminophen) API.
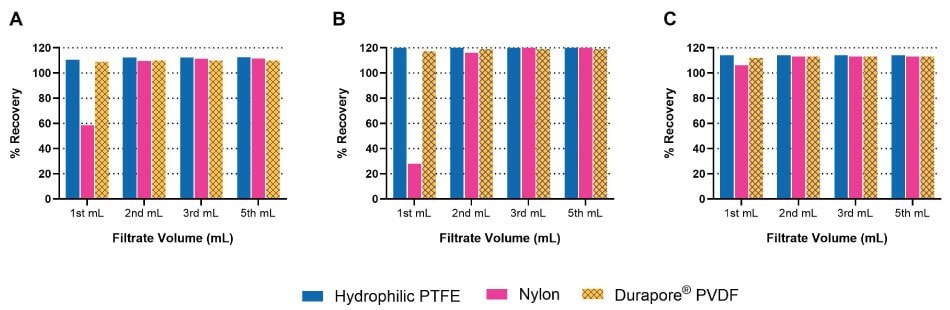
Figure 1.Recovery of A) acetaminophen (278 µg/mL), B) acetyl salicylic acid (278 µg/mL), and C) caffeine (72 µg/mL) from a multi-component migraine formulation using different membrane filters. Recovery was measured after the 1st, 2nd, 3rd, and 5th mL of filtrate.
As can be seen in Figure 1, both acetaminophen and acetyl salicylic acid bind strongly to Millex® filters containing nylon membrane but not to Millex® filters containing hydrophilic PTFE or Durapore® PVDF membranes. On the other hand, caffeine doesn’t show binding to any of the three membranes selected in this study.
Both hydrophilic PTFE and Durapore® PVDF membranes have very few functional groups that can interact with various analytes, thereby leading to low analyte binding and subsequently high recovery. Nylon membrane contains amino and carboxylic acid functional groups as well as amide bonds which can interact with acidic or basic analytes through electrostatic and hydrogen bonding interactions, leading to high analyte binding and low recovery. Analyte recovery with nylon syringe filters can be improved by simply saturating the syringe filter with the sample, thereby saturating the binding sites present on the membrane. This can be clearly seen in Figure 1 for second, third, or fifth 1 mL of filtrate.
Analyte Concentration
Quantitative analyte recovery can be obtained after the filter is fully saturated with the analyte. When the membrane filter is saturated, no further analyte binding is observed because syringe filters have limited surface area as well as a limited number of functional sites that enable analyte binding. This saturation point is dependent on analyte concentration. Generally, as the analyte concentration decreases the volume needed to fully saturate the filter increases.
Figure 2 shows the effect of analyte concentration on analyte binding and subsequent recovery of naproxen from a formulation. Three different concentrations of naproxen ranging from 244 ppm to 2.4 ppm were filtered through hydrophilic PTFE Millex® syringe filters and filtrate was analyzed by HPLC. As can be seen in Figure 2, no analyte binding was observed for the high concentration of naproxen (244 ppm), even without any filter saturation (no discard volume). At lower concentrations, slightly lower recovery was obtained for first 1 mL of filtrate due to incomplete saturation of the syringe filter but quantitative recovery can be obtained for third or fifth mL of filtrate (indicating filter saturation). Since hydrophilic PTFE membrane generally shows low analyte binding, this concentration effect was subtle, but still can be significant if the recovery specification was very narrow.
This study was conducted with both 0.45 µm and 0.2 µm Millex® hydrophilic PTFE syringe filters, with very similar results obtained (Figure 2). Membrane pore size did not significantly impact naproxen saturation of syringe filters. This concentration effect is membrane- and analyte-dependent and hence, during filter validation studies, discard volume should be selected carefully depending on analyte concentration.
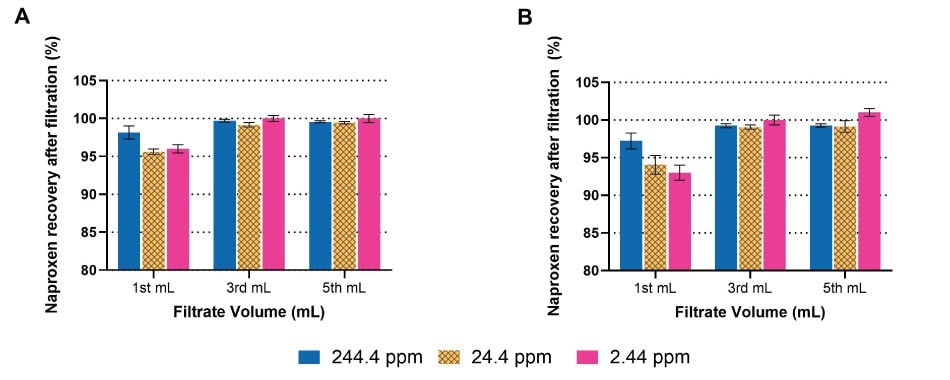
Figure 2.Effect of naproxen concentration of analyte recovery using A) 0.45 µm and B) 0.2 µm Millex hydrophilic PTFE syringe filters.
Preparation of Sample and Standards
Processing conditions of standard and sample can impact analyte binding and subsequent analyte recovery. Good lab practices suggest that the sample and standard should undergo the same processing to avoid any impact of processing conditions on analyte recovery. The example below shows what can happen when there are differences in sample preparation for experimental samples and standards. The assay is analyte recovery from blend uniformity samples. The sample was dissolved in the solvent mixture and then filtered using Durapore® PVDF Millex® syringe filter. Five milliliters of filtrate volume were discarded before sample was collected for HPLC analysis. The standard was processed using three different conditions:
- Standard 1: Standard created following the customer protocol (unfiltered).
- Standard 2: Standard created by dissolving pure compound in the solvent mixture followed by filtration using various Millex® syringe filters with 5 mL filtrate discarded prior to HPLC (filtered).
- Standard 3: Using blend uniformity sample dissolved in the solvent mixture and centrifuging prior to HPLC analysis (centrifuged sample).
Results are shown in Table 3. When Standard 2 was used for recovery calculations, consistent and quantitative recovery was obtained for all the syringe filters tested. This is expected since both the standard and sample undergo the same processing method, thereby reducing any impact of analyte binding on recovery.
On the other hand, when Standard 1 or 3 was selected for recovery calculations, a negative impact was observed on sample recovery due to analyte binding. This effect was more pronounced with Millex® Durapore® PVDF syringe filters compared to Hydrophilic PTFE Millex® syringe filter. Some lot-to-lot variability was also observed for using the Durapore® PVDF syringe filter.
Volumetric Sample Recovery
In most cases, pharmaceutical QC tests are not sample-limited and volumetric sample recovery may not largely impact sample availability. However, when calculating analyte binding, volumetric sample recovery plays an important role since the volume required for filter saturation needs to account for the volumetric retention of syringe filters.
Figure 3 shows sample volume recovered when 2 mL water was filtered through various 25 mm and 33 mm syringe filters. Of all the filters tested, Millex® filters (33 mm diameter) allowed for maximum sample volume to be recovered (~1.4 mL) when filtering 2 mL water, whereas the polypropylene membrane-based syringe filter (25 mm diameter) only recovered 0.6 mL of sample volume. This volume retention is dependent on the filter design and is not largely impacted by membrane pore size. With the other three membrane filters tested, approximately 1 mL of sample was retained within the syringe filter.

Figure 3.Volume recovery after filtration of 2 mL water through various syringe filters. The hydrophilic PTFE Millex® filters are 33 mm in size, whereas all the other syringe filters (from vendor B, C, D and E) were 25 mm in size. Membranes tested include hydrophilic PTFE, regenerated cellulose (RC), and polypropylene (PP).
Conclusions
- Filter validation is a critical part of various pharmaceutical analytical QC tests, and various filter parameters need to be taken into consideration during method validation. Since accurate analyte quantitation is required in these QC tests, analyte binding is an important factor to consider during filter validation.
- Membrane and analyte physico-chemical properties have the largest impact on analyte recovery. Low analyte concentration exacerbates this effect.
- Membrane pore size has limited impact on analyte recovery, but pore size selection is dictated by downstream analytical technique.
- Using different processing techniques for sample and standard impacts analyte recovery, and hence it is preferable for standard to undergo the same process as the sample.
- Differences in syringe filter designs led to differences in volumetric recovery. This can be critical for small sample volumes and filter saturation.
References
To continue reading please sign in or create an account.
Don't Have An Account?