All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C26H41N3O5
CAS Number:
Molecular Weight:
475.62
UNSPSC Code:
12352200
NACRES:
NA.32
Recommended Products
Assay
≥98%
Quality Level
solubility
DMSO or DMF: 25 mg/mL
storage temp.
−20°C
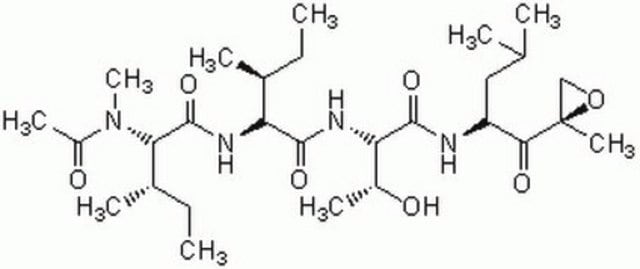
SMILES string
CC(C)C[C@@H](C(N[C@H](C(N[C@H](C=O)CC(C)C)=O)CC(C)C)=O)NC(OCC1=CC=CC=C1)=O
Application
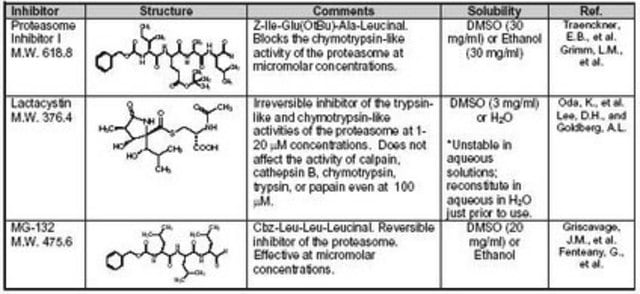
(R)-MG132 has been used in ubiquitination assay and is used as a proteasome inhibitor.
Biochem/physiol Actions
MG132 (carbobenzoxy-Leu-Leu-leucinal) is a tri-peptide aldehyde. It possesses antitumor activity and boosts cytostatic/cytotoxic effects of chemo- and radiotherapy. (R)-MG132 is a potent, membrane-permeable proteasome inhibitor. It can inhibit proteasome activity in lysates of J558L multiple myeloma cells and EMT6 breast cancer cells. The (R)-MG132 stereoisomer is a more effective inhibitor of chymotrypsin-like (ChTL), trypsin-like (TL), and peptidylglutamyl peptide hydrolyzing proteasome (PGPH) activities than the (S)-MG132.
Physical form
crystalline solid or supercooled liquid
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Studies of the synthesis of all stereoisomers of MG-132 proteasome inhibitors in the tumor targeting approach.
Mroczkiewicz M, et al.
Journal of Medicinal Chemistry, 53(4), 1509-1518 (2010)
Alternative promotion and suppression of metastasis by JNK2 governed by its phosphorylation.
Hu S, et al.
Oncotarget, 8(34), 56569-56569 (2017)
A Mad2-Mediated Translational Regulatory Mechanism Promoting S-Phase Cyclin Synthesis Controls Origin Firing and Survival to Replication Stress.
Gay S, et al.
Molecular Cell, 70(4), 628-638 (2018)
J Yang et al.
Oncogene, 36(34), 4828-4842 (2017-04-11)
PIM1 is a proto-oncogene, encoding a serine/threonine protein kinase that regulates cell proliferation, survival, differentiation and apoptosis. Previous reports suggest that overexpression of PIM1 can induce cellular senescence. However, the molecular mechanism underlying this process is not fully understood. Here
Determination of H-ATPase Activity in Arabidopsis Guard Cell Protoplasts through H-pumping Measurement and H-ATPase Quantification.
Yamauchi S and Ken-ichiro S
Plant Physiology (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service