Antibody Fragmentation with Pepsin Digestion
Pepsin is an endopeptidase that breaks down proteins into smaller peptides. It is produced in the gastric chief cells of the stomach lining and is required for protein digestion, one of the main digestive enzymes in the digestive systems of humans and many other animals. Pepsin is a member of the Peptidase A1 family and exhibits preferential cleavage for hydrophobic, aromatic regions in the protein.
Pepsin Digestion of Antibodies for Preparation of F(ab’)2
Pepsin is widely used in antibody research for digesting immunoglobulins, bivalent antigen-binding fragments (F(ab’)2), and additional proteins. It can be used to separate the F(ab’)2 region from the Fc domain of various immunoglobulin isotypes, such as IgG and IgA. Unlike Papain, pepsin cleavage results in both chains of the Fab domain connected as cleavage occurs toward the C-terminal side of the disulfide bonds connecting the chains, as shown in Figure 1.

Figure 1.Schematic illustration of antibody fragmentation with pepsin and papain.
Isolation of F(ab’)2 is useful in immunology research applications as it maintains the avidity of a bivalent antibody without the complement Fc receptor binding function of the Fc domain 1. F(ab’)2 isolation comes with several additional advantages, including improved stability compared to smaller fragments (scFv), and the small size enables better permeability and easier access to ligands. Improving the reliability and efficiency of Pepsin antibody digestion, our experts calibrated the pepsin digestion protocol of monoclonal and polyclonal antibodies with pepsin (Cat No: P6887) to reach rapid, simple, and complete digestion.

Figure 2.Time course of monoclonal antibodies with P6887.

Figure 3.Time course of polyclonal antibodies with P6887.
Data obtained using pepsin digestion protocol suggests rapid digestion of monoclonal and polyclonal antibodies with Pepsin (Cat No: P6887) while retaining F(ab’)2 specificity (see Figure 4).
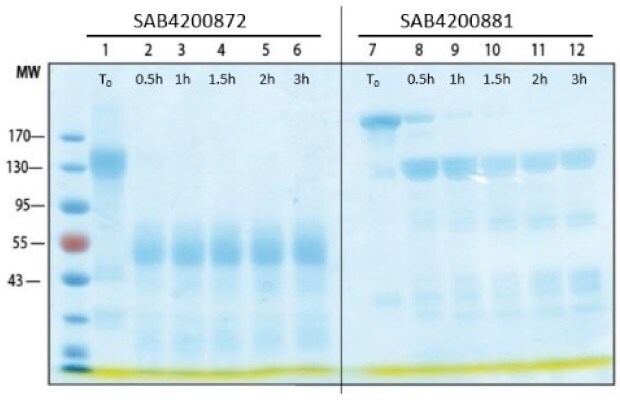
Figure 4.Digestion of polyclonal and monoclonal antibodies with Pepsin. SDS-PAGE analysis of pepsin-treated antibody for different time periods. SAB4200872: Anti-SARS-CoV-2-Spike protein C-terminal antibody produced in Rabbit; SAB4200881: Anti-β-lactoglobulin antibody, Mouse monoclonal.
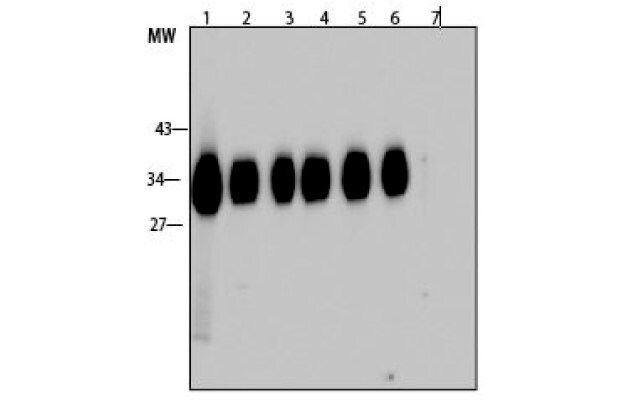
Figure 5.Western blot analysis of pepsin-treated antibody for different time periods is active and binds its target. Lanes 1-6 are corresponding to the lanes in the SDS-PAGE above. Lane 7: Negative control; SAE1000: SARS-CoV-2 Receptor Binding Domain; L3908: β-lactoglobulin.
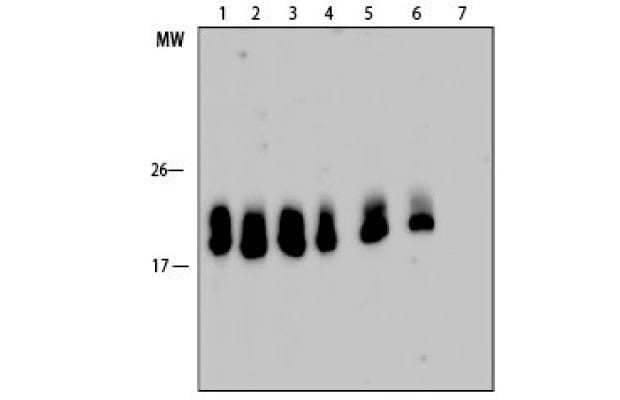
Figure 6.Western blot analysis of pepsin-treated antibody for different time periods is active and binds its target. Lanes 1-6 are corresponding to the lanes in the SDS-PAGE above. Lane 7: Negative control; SAE1000: SARS-CoV-2 Receptor Binding Domain; L3908: β-lactoglobulin.
Pepsin Digestion Materials |
|---|
Pepsin Digestion Protocol
- Concentrate the antibody to 25-35 mg/ml.
- Exchange buffer to 0.1M sodium citrate buffer pH 3.5. Note: Dialysis overnight at 4 °C has been tested and is sufficient
- Dissolve 10 mg pepsin (P6887) in 1 ml 0.1M sodium citrate buffer pH 3.5.
- Add the dissolved pepsin to the antibody solution. Pepsin should be added in a proportion of 3% (w/w) to the target antibody. (If processing 10 mg of Antibody, add 30ug of pepsin or 30L of the pepsin solution)
- Incubate at 37 °C for 1-2 hours.
- Stop the reaction by titration with 2M TRIS-base to final pH 8.0.
- Dialyze the antibody against 0.01M PBS pH 7.2-7.4 (add 15mM Na Azide for preservation) or your preferred buffer, overnight at 4 °C.
References
To continue reading please sign in or create an account.
Don't Have An Account?