905313
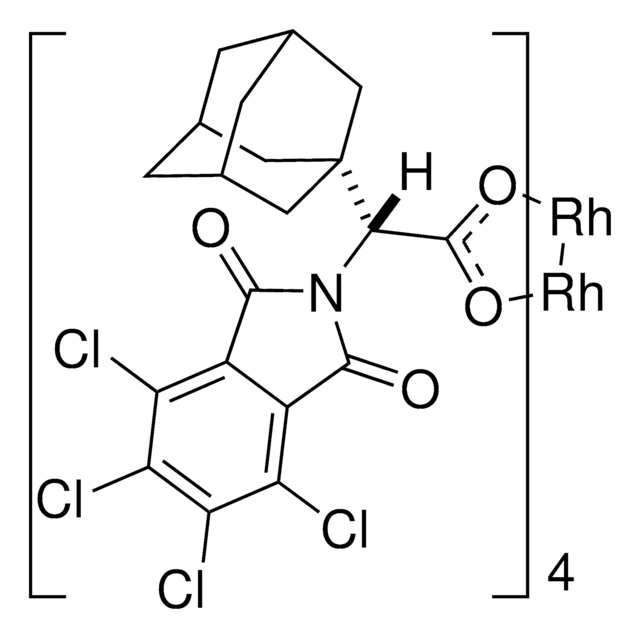
Rh2(S-TCPTAD)4
Synonym(s):
Davies dirhodium catalyst
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C80H64Cl16N4O16Rh2
CAS Number:
Molecular Weight:
2110.44
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
form
powder or crystals
Related Categories
Application
This dirhodium catalyst developed by the Davies lab can form C-C bonds at the most accessible tertiary C-H position with control of both regioselectivity and absolute configuration.
Other Notes
Formation of Tertiary Alcohols from the Rhodium-Catalyzed Reactions of Donor/Acceptor Carbenes with Esters
Harnessing the β-Silicon Effect for Regioselective and Stereoselective Rhodium(II)-Catalyzed C-H Functionalization by Donor/Acceptor Carbenes Derived from 1-Sulfonyl-1,2,3-triazoles
Site-selective and stereoselective functionalization of non-activated tertiary C-H bonds
Harnessing the β-Silicon Effect for Regioselective and Stereoselective Rhodium(II)-Catalyzed C-H Functionalization by Donor/Acceptor Carbenes Derived from 1-Sulfonyl-1,2,3-triazoles
Site-selective and stereoselective functionalization of non-activated tertiary C-H bonds
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hengbin Wang et al.
Chemical science, 4(7), 2844-2850 (2013-09-21)
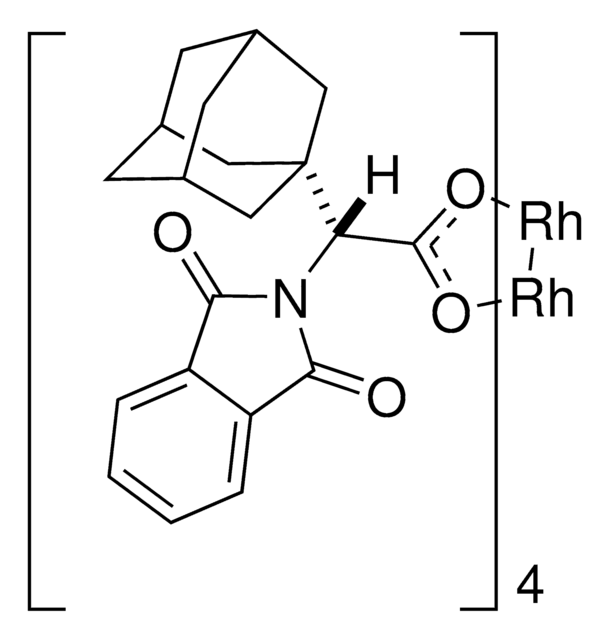
The rhodium-catalyzed reaction of electron-deficient alkenes with substituted aryldiazoacetates and vinyldiazoacetates results in highly stereoselective cyclopropanations. With adamantylglycine derived catalyst Rh2(S-TCPTAD)4, high asymmetric induction (up to 98% ee) can be obtained with a range of substrates. Computational studies suggest that
Liangbing Fu et al.
Organic letters, 20(8), 2399-2402 (2018-04-12)
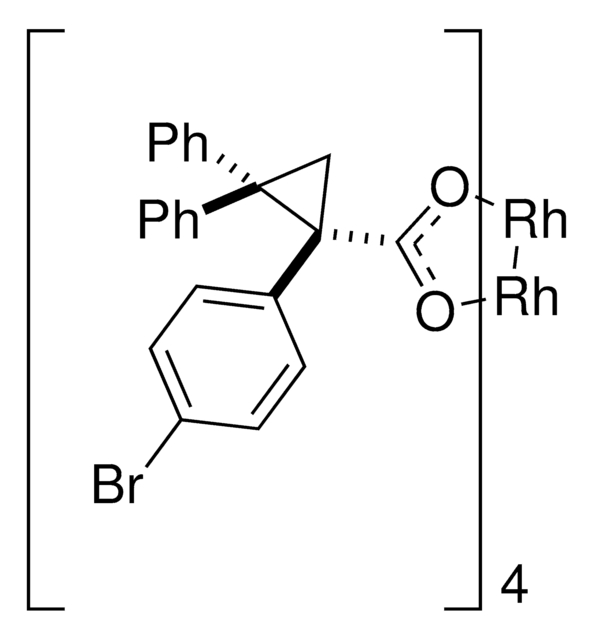
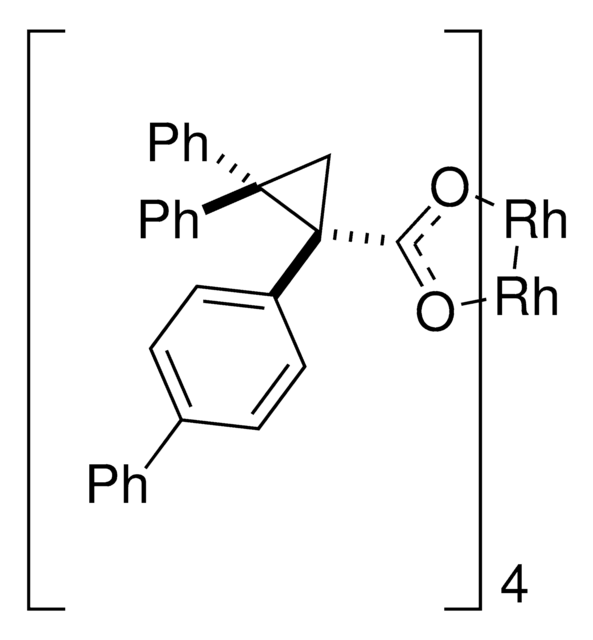
Rhodium(II)-catalyzed reactions between isopropyl acetate and trichloroethyl aryldiazoacetates result in the formation of oxirane intermediates that ring open under the reaction conditions to form tertiary alcohols. When the reaction is catalyzed by the dirhodium tetrakis(triarylcyclopropanecarboxylate) complex, Rh2( S-2-Cl,4-BrTPCP)4, the tertiary
Atsushi D Yamaguchi et al.
Journal of the American Chemical Society, 137(2), 644-647 (2015-01-07)
Syntheses of dictyodendrins A and F have been achieved using a sequential C-H functionalization strategy. The N-alkylpyrrole core is fully functionalized by means of a rhodium(I)-catalyzed C-H arylation at the C3-position, a rhodium(II)-catalyzed double C-H insertion at the C2- and
Enantioselective intramolecular aza-spiroannulation onto benzofurans using chiral rhodium catalysis.
Shibuta T, et al.
Heterocycles, 89, 631-639 (2014)
Shigeki Sato et al.
Chemical communications (Cambridge, England), (41), 6264-6266 (2009-10-15)
A versatile, highly enantiocontrolled entry to the spiro-beta-lactam core of chartellines has been developed by expanding the scope of oxidative nitrogen atom transfer methodology based on chiral Rh-nitrenoid species.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionic acid)] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)